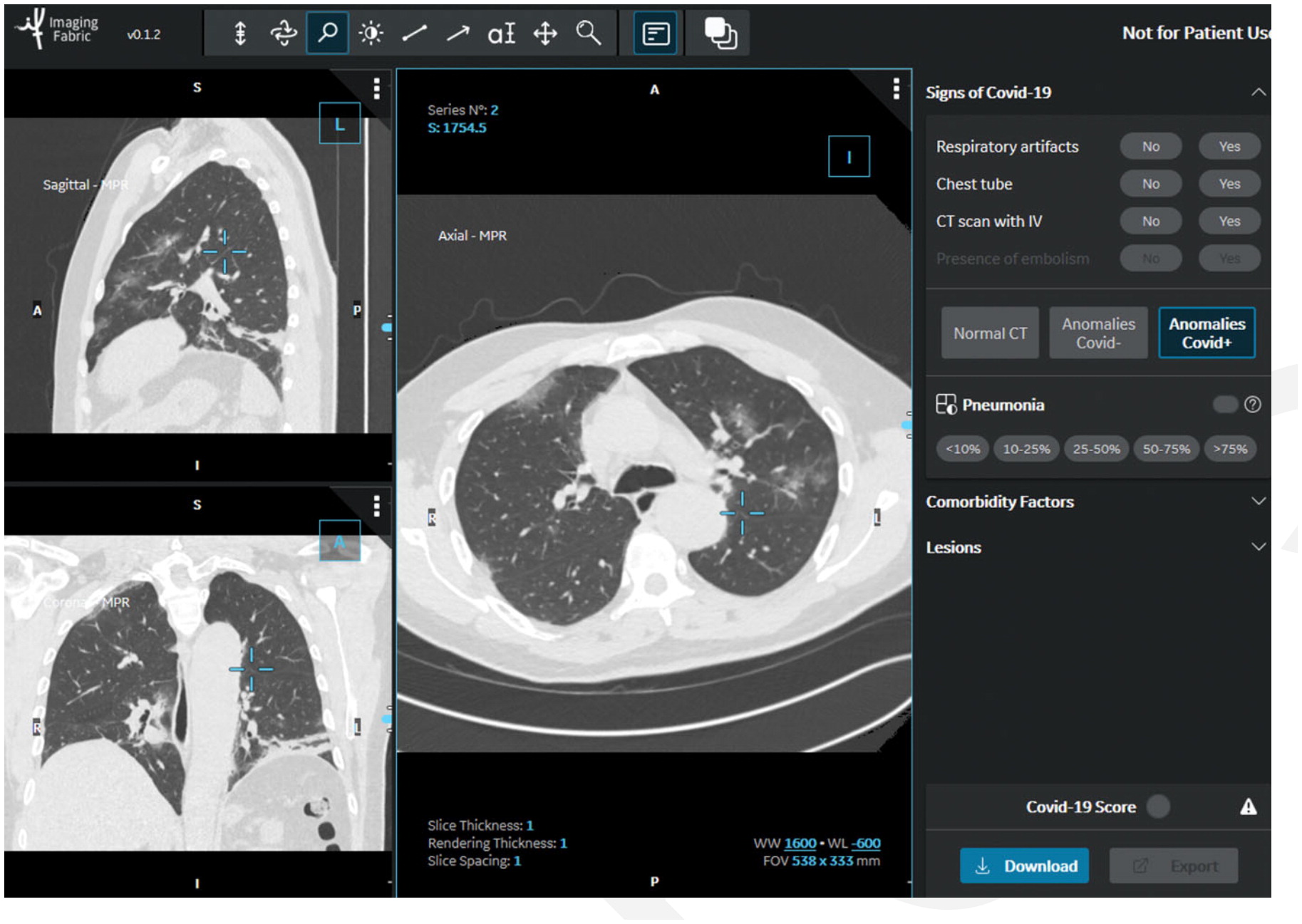
In the STOIC study, readers classified CT exams as COVID positive, COVID negative or normal. The readers had access to the CT scans using a 3-D image visualization web application, allowing scrolling through the entire lung volume in the coronal, sagittal or axial transverse plane. The CT scan shown here has been classified as COVID positive due to the presence of bilateral ground glass opacities and absence of features such as mucoid impaction, bronchiolar nodules, segmental or lobar consolidation. Image courtesy of RSNA
In a new study published in Radiology of more than 10,000 patients, researchers found that chest computed tomography (CT) was 80 percent accurate in diagnosing COVID-19 pneumonia and predictive of death or the need for intubation. Accuracy increased to 86 percent after five days of symptoms. The extent of pneumonia on chest CT was the best predictor of severe outcome (intubation or death) at one month.
Early experience in China and some small studies published at the beginning of the pandemic in early 2020 found CT utility for the modality in confirming COVID infections based on lung scans. Prior to widely available PCR testing for COVID, it was suggested CT could be used to detect the virus, in some cases diagnosing the virus before the patient tested positive using a PCR test. Once the virus reached the United States, there was hesitation to use CT because of its cost, radiation dose, debate over accuracy and the concerns about the need to decontaminate scanners after each use, which adds to room turnaround times.
However, this new, large-scale, multicenter observational retrospective cohort study confirms the early findings that CT has a high level of accuracy for diagnosing COVID. This study helps answer the conflicting data from smaller, previous studies regarding the diagnostic performance of chest CT for COVID-19 pneumonia.
The STOIC (Study of Thoracic computed tomography In COVID-19) project aimed to build a dataset of at least 10,000 CT scans from individuals with suspected COVID-19 pneumonia, evaluated during the first wave of the SARS-CoV-2 pandemic in France.
Using RT-PCR as the reference standard in 10,735 patients with suspected COVID-19 pneumonia, CT was 80 percent accurate and was also predictive of death or need for intubation, the study found.
Researchers looked at data from patients who presented at the emergency departments of 20 French university hospitals between March 1 and April 30, 2020. It included patients who underwent both chest CT and RT-PCR for suspected COVID-19 pneumonia. CT images were analyzed blinded to initial reports, RT-PCR, demographic characteristics, clinical symptoms and outcome.
Of the 10,735 patients, 6,448 (60 percent) had a positive RT-PCR result. With RT-PCR as reference, the sensitivity and specificity were 80.2 percent and 79.7 percent, respectively, with strong agreement between junior and senior radiologists. Of all the variables analyzed, the extent of pneumonia on CT was the best predictor of severe outcome at one month. A score based solely on clinical variables predicted a severe outcome when it also included the extent of pneumonia and coronary calcium score on CT.
Readers classified CT scans as positive or negative for COVID-19, based on criteria published by the French Society of Radiology. Multivariable logistic regression was used to develop a model predicting severe outcome (intubation or death) at one-month follow-up in subjects positive for both RT-PCR and CT, using clinical and radiological features. If the first RT-PCR result was negative, but turned out to be positive in the following seven days after CT, the patient was considered as positive for SARS-CoV-2 infection. CT studies in China often found evidence of COVID pneumonia in the lungs from early infection prior to positive PCR tests, and this was also found in the study.
Increased CT Specificity for COVID and Standardized Data Collection
"In our study, CT specificity, although insufficient for a confident diagnosis, was much higher than that of the first reports," researchers wrote.[2]
A first review based on studies mainly from Asian countries reported a pooled CT specificity of only 18.1 percent.[3] Even though an update of this first review reported a pooled specificity of 61.1 percent,[4] the authors estimated that the heterogeneity and insufficient quality of the included studies limited their possibility to draw conclusions and highlighted the need to conduct future diagnostic accuracy studies that should predefine positive imaging findings.
The structured reporting of COVID-19 pneumonia proposed by the French Society of Radiology, used in this study, requires that bronchiolar nodules, mucoid impactions and focal consolidation should be absent for diagnosis.[5] Other proposals for structured reporting also include CT features favoring diagnoses other than COVID-19 pneumonia.[6-8] CORADS (COVID-19 Reporting and Data System) 2 category corresponds to a low level of COVID-19 suspicion when centrilobular nodules, lobar or segmental consolidation or lung cavitation are observed. COVID-RADS includes pulmonary nodules and airway secretions as inconsistent with COVID-19.[7] The Radiological Society of North America (RSNA) consensus document suggested one atypical appearance category: bronchiolar nodules or cavitation.[8]
As recommended by the American College of Radiology [9] RT-PCR is the only specific method of diagnosis even though it has imperfect sensitivity.[10] The first RT-PCR showed a sensitivity of 95.8 percent in this study, but not all negative tests were repeated. When RTPCR was negative and CT was interpreted as positive, 39.3 percent of the initially negative RTPCR results were positive on repeat testing, highlighting the need for further testing in cases of discrepancies, the authors wrote.
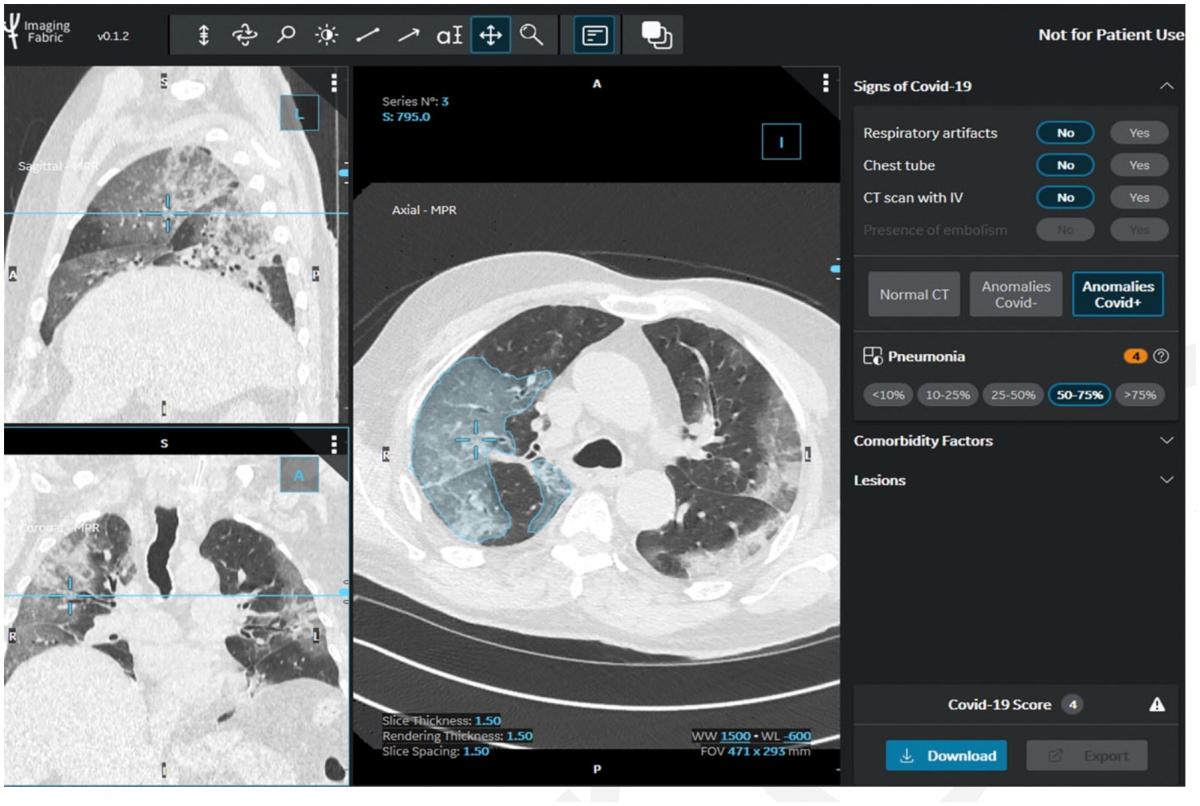
Visual quantification of COVID lung disease extent from the STOIC study. The readers had to visually quantify the extent of COVID-19 pneumonia on a five-point scale. This image is estimated to be between 50-75 percent. Readers were also asked to manually contour COVID-19 pneumonia (area in blue in the right lung) on at least two CT images to later train deep learning algorithms for automated quantification of disease extent. Image courtesy of RSNA
All RSNA COVID-19 articles can be found at Special Focus: COVID-19. Additional COVID-19 resources may be found at RSNA.org/COVID-19.
Related Radiology COVID-19 Content:
Medical AI Models Rely on 'Shortcuts' That Could Lead to Misdiagnosis of COVID-19
CT Provides Best Diagnosis for Novel Coronavirus (COVID-19)
SNMMI Image of the Year: PET Imaging Measures Cognitive Impairment in COVID-19 Patients
Cardiac MRI Effective in Detecting Asymptomatic, Symptomatic Myocarditis in Athletes
PHOTO GALLERY: How COVID-19 Appears on Medical Imaging
VIDEO: How to Image COVID-19 and Radiological Presentations of the Virus — Interview with Margarita Revzin, M.D.
How Does COVID-19 Appear in the Lungs?
Find more radiology related COVID news and video
References:


 January 28, 2026
January 28, 2026 









