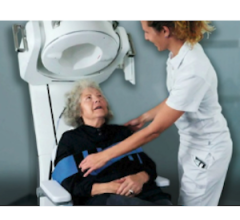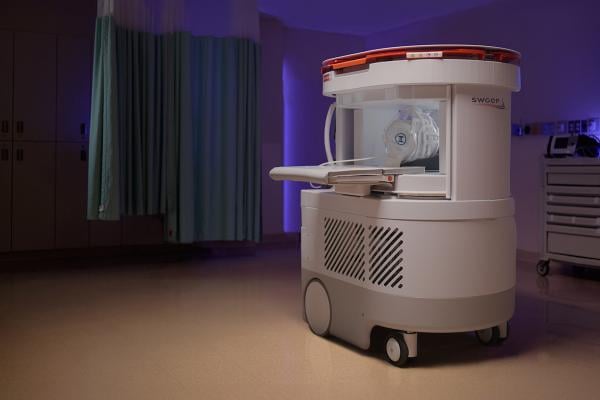
Jan. 20, 2026 — Hyperfine, the developer of the first FDA-cleared AI-powered portable MRI system for the brain — the Swoop system — has announced results from the NEURO PMR study, presented by Dr. Laszlo Mechtler at the American Society of Neuroimaging (ASN) meeting in San Juan, Puerto Rico on Jan. 16, 2026.
NEURO PMR (Neurological Evaluation in the Office with Portable MR) is the first prospective, multi-center, real-world study to evaluate the clinical utility and patient experience of portable MRI compared to standard-of-care MRI in outpatient neurology clinics. Two sites — DENT Neurologic Institute and Texas Neurology — enrolled 125 patients with neurologic conditions commonly seen in the outpatient setting, including headache, dementia, multiple sclerosis follow-up, and tumor surveillance. Patients received brain imaging on both the portable Swoop system (0.064T) and a conventional high-field MRI (primarily 3T), enabling direct modality comparisons.
Portable MRI demonstrated 92% concordance with standard MRI in identifying the presence or absence of intracranial pathology during a blinded review by independent neuroradiologists. In unblinded paired image reviews incorporating clinical history, concordance increased to 98%, as assessed by a neurologist and neuroimager. Patients expressed a strong preference for portable MRI, reporting that they were four times more likely to choose it over standard MRI. Across all experience measures — including comfort, anxiety, claustrophobia, noise and overall satisfaction — portable MRI was rated superior to standard MRI (p<0.0001). Trained clinical staff successfully operated the system within neurology offices without the need for MR technologists, highlighting its safe and simple workflow.
“From a clinical perspective, I have been impressed by the image quality delivered by the Swoop system. The study results give me confidence in its ability to detect structural abnormalities encountered in routine neurological care,” said Laszlo Mechtler, MD, Chief Medical Officer at DENT Neurologic Institute and Principal Investigator of the NEURO PMR study. “Portable MRI represents a groundbreaking innovation for neurology clinics, complementing tools like EEG and ultrasound to expand diagnostic capabilities at the point of care. Neuroimaging is not merely an ancillary test but an extension of the neurological examination — expanding access to this technology will put imaging back into the neurologist’s hands and meaningfully improve the quality of patient care.”
The Swoop system addresses challenges that have historically prevented neurology practices from bringing MRI on-site. It is portable, fits in an exam room, requires no specialized sitting, shielding, or helium, and plugs into a standard outlet. Scans can be performed by existing clinic staff and are eligible for reimbursement under existing brain MRI CPT codes after site accreditation.
"The NEURO PMR results validate the transformative role portable MRI can play in expanding imaging beyond traditional hospital settings,” said Chi Nguyen, Senior Vice President of Office & Community Business at Hyperfine. “By removing the economic and operational barriers of conventional MRI, the Swoop system empowers neurology practices to deliver safe and convenient imaging in the clinic. This is a win for physicians, who gain timely diagnostic insights; a win for patients, who enjoy a better imaging experience; and a win for the healthcare system, which benefits from efficient, cost-effective care delivery."
For more information about the Swoop system, please visit HyperfineMRI.com.


 March 02, 2026
March 02, 2026