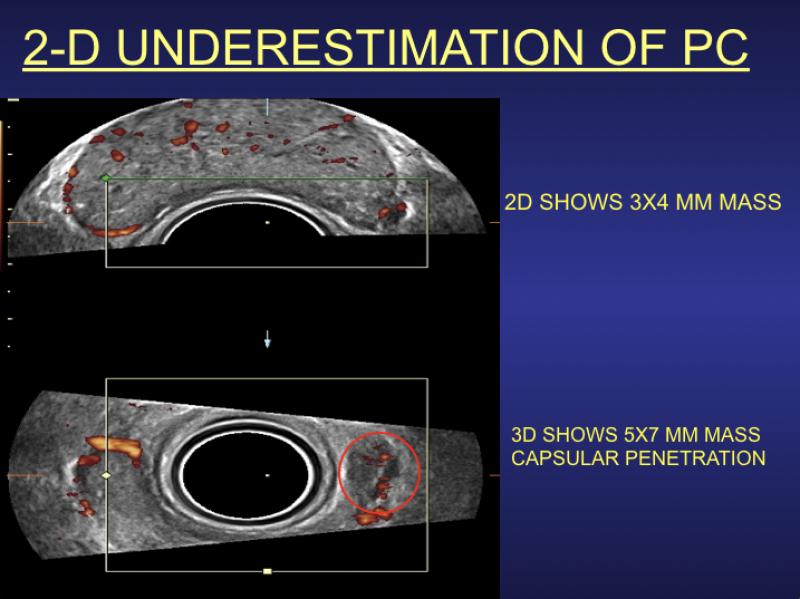
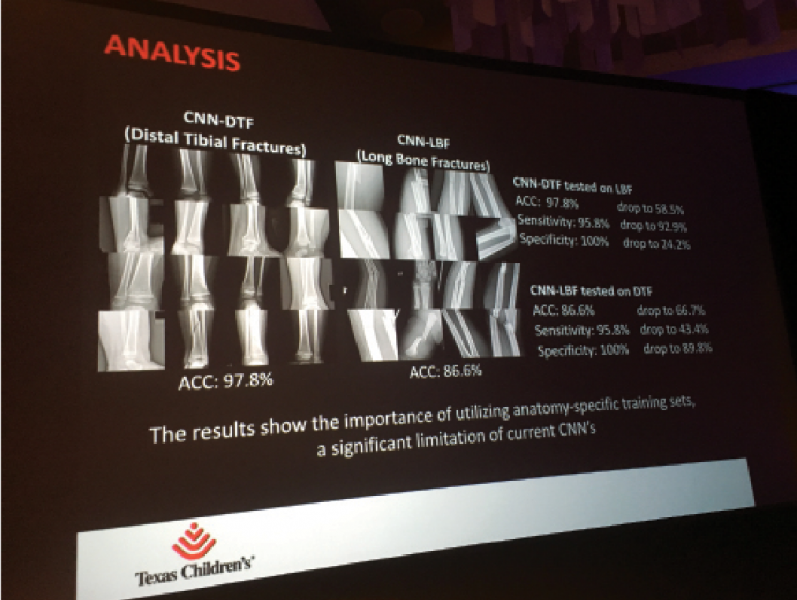
The use of smart algorithms has the potential to make healthcare more efficient. Sarah Eskreis-Winkler, M.D., presented ...
Pragmatism from cybersecurity to enterprise imaging was in vogue at the 2019 meeting of the Society of Imaging ...
Xifin announced the launch of the next evolution of its laboratory information system (LIS), Xifin LIS 6. The expanded platform features enhancements that support high-complexity, high-volume labs, as well as new integrated capabilities available through strategic partners, including artificial intelligence (AI)-powered digital pathology workflow and genomic data interpretation, automated prior authorization and test utilization decision support.Xifin announced the launch of the next evolution of its laboratory information system (LIS), Xifin LIS 6. The expanded platform features enhancements that support high-complexity, high-volume labs, as well as new integrated capabilities available through strategic partners, including artificial intelligence (AI)-powered digital pathology workflow and genomic data interpretation, automated prior authorization and test utilization decision support.
Radiology departments have many different needs and face a wide variety of challenges that can impact their departments ...
A new clinical guideline from the American Society for Radiation Oncology (ASTRO) provides recommendations on the use of radiation therapy to treat patients diagnosed with pancreatic cancer. Recommendations include when radiation treatments are appropriate, as well as the optimal dosing, timing and fractionation for these treatments. The guideline, which also outlines strategies to prevent and mitigate common side effects of pancreatic radiation therapy, is published online in Practical Radiation Oncology, the clinical practice journal of ASTRO.
In molecular radiotherapy (MRT) treatment of the thyroid, existing single photon emission computed tomography (SPECT) ...
September 5, 2019 — An ahead-of-print article published in the December issue of the American Journal of Roentgenology ...
Despite decades of progress in breast imaging, one challenge continues to test even the most skilled radiologists ...
Heidelberg University Hospital, Germany, has treated its first patient using Monte Carlo photon dose planning in RayStation. The decision to go clinical was made after a thorough validation of the functionality. The first patient, who has cervical cancer involving the lymph nodes, was recently treated.
The global rise in chronic disease has significantly increased demand for diagnostic imaging procedures, and in turn ...
Extracapsular extension (ECE) is a major clinical indicator for brachytherapy or external beam treatment of prostate ...
Bayer Radiology’s Barbara Ruhland and Thom Kinst discuss how radiology departments can address the many different ...
Iridium Cancer Network consists of all seven hospitals in the Antwerp region, closely collaborating regarding radiation ...
Burnout has become a popular buzzword in today’s business world, meant to describe prolonged periods of stress in the ...
Doctors and technologists are exposed to on-the-job radiation often on a daily basis. It is critical to be aware of how ...
eHealth Saskatchewan plays a vital role in providing IT services to patients, health care providers, and partners such ...
In healthcare, critical systems are being used to deliver vital information and services 24x7x365. Clinicians demand ...
OSF HealthCare in Peoria, Ill., recently added a new Carestream DRX-Evolution Plus System to its portfolio of Carestream imaging solutions across the integrated health system’s diverse operations. The imaging portfolio include Carestream DRX-Revolution mobile X-ray systems and Carestream DRX-Ascend systems, along with Carestream DRX-Plus detectors.
LAP LLC announced its Apollo MR3T positioning laser for radiation therapy has received U.S. Food and Drug Administration (FDA) 510(k) approval. The magnetic resonance (MR)-compatible room laser is currently the only product of its class approved the FDA, according to the company.
Global Diagnostics Australia (GDA), a subsidiary of the Integral Diagnostics Group (IDX), has adopted artificial intelligence (AI) applications into its radiology workflow, and is one of the first diagnostic imaging companies in Australia to do so. GDA has partnered with AI company Aidoc to incorporate their algorithms into GDA's care management pathway. These AI applications will expedite patient diagnosis and treatment for several head, neck and chest conditions.
Radiology’s history dates back to 1895 when Wilhelm Roentgen discovered X-rays. The very first image taken was of his ...
Help may be on the way for people who might lose contact with reality through a psychotic disorder, such as schizophrenia.
Modus QA is proud to offer the world's first MR-safe Motion QA phantom for simulation, planning and delivery ...
Despite a broad campaign among physician groups to reduce the amount of medical imaging, use rates of various scans have continued to increase in both the U.S. and Ontario, Canada, according to a new study. A recent reacceleration in the growth of imaging concerns researchers because it is widely believed to be overused. The study of more than 135 million imaging exams was conducted by researchers at UC Davis, UC San Francisco and Kaiser Permanente.

 September 06, 2019
September 06, 2019