June 19, 2019 — Zebra Medical Vision announced it has received its third U.S. Food and Drug Administration (FDA) 510(k) clearance for the company's HealthICH product. The new clearance covers an artificial intelligence (AI) alert for intracranial hemorrhage (ICH), based on head computed tomography (CT) scans.
The latest cleared product automatically identifies suspected internal brain bleeds based on non-contrast head CTs for triaging, significantly reduces turnaround time and increases the radiologists' confidence in their diagnosis. The overall Multi-Modality AI Triage Solution by Zebra-med is a first of its kind in the market, according to the company, that provide alerts for both CT scans and X-rays. It currently addresses two acute conditions: intracranial hemorrhages (head CTs) and pneumothorax (chest X-rays), which also received FDA clearance in the past month. Both triage solutions and others, such as the FDA-cleared calcium scoring product, are part of the AI1 "all-in-one" bundle that provides hospitals with a growing amount of AI tools at a fixed annual price and consistent service and support.
Intracranial hemorrhage accounts for about 10-20 percent of all strokes, and can occur as a result of traumatic injury (falls, crashes, etc), ruptured arteries (aneurysms), stroke or cancer. It can result from injury within a vein or artery, or a combination of the two. Regardless of the cause, the timely and accurate diagnosis of active bleeding is essential for the successful care of these patients. Intracranial hemorrhage — or the common phrasing, brain bleed — is a devastating disease, with a high 30-day mortality rate that ranges from 35-52 percent. It is estimated that about half of this mortality occurs within the first 24 hours, emphasizing the importance of early detection and effective treatment in the emergency department (ED).
The financial impact of early detection would be enormous given the fact that stroke cost the U.S. alone $34 billion annually, including the cost of healthcare services, medications and lost productivity. The overall incidence of spontaneous ICH worldwide is 24.6 people in every 100,000 , with approximately 40,000-67,000 cases per year in the United States.
Zebra-Med's intracranial hemorrhage triage solution can provide early detection of people who may have experienced a brain bleed. The algorithm is comprised of a tailor-made neural network architecture designed to identify intracranial hemorrhage while tackling several challenges, such as relatively small bleed sizes and common artifacts (metal, motion artifacts) seen within the brain.
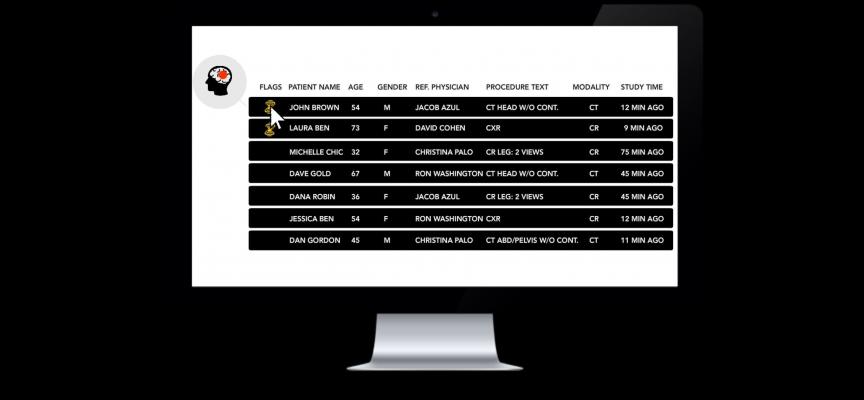
In busy ED environments, teleradiology services and other healthcare organizations that integrate Zebra-Med's AI1 solution, radiologists can address these prioritized scans in a timely manner. Scans are forwarded automatically to the Zebra-Med server for analysis. The server then sends an alert of the positive cases directly to the worklist for prioritizing, thus saving physicians more than 80 percent of the time taken to reach the acute condition.
The triage of intracranial hemorrhage product is supported by Zebra-med's recent research results, “Improved Intracranial Hemorrhage Classification using Deep Multi-task Learning.” published at the Society for Imaging Informatics in Medicine (SIIM) conference in 2018. The results demonstrated how AI-based first read can achieve markedly high levels of intracranial hemorrhage detection and classification.
Keith White, M.D., medical director of imaging services at Intermountain Healthcare stated, "We are working to improve our ability to quickly identify and respond to potentially dangerous clinical conditions through deployment of Zebra Medical Vision's intracranial hemorrhage solution"
Stanley Lu, M.D., radiologist and head of neuroradiology at Monmouth Medical Center, part of RWJBarnabas Health, stated, "We have deployed Zebra Medical Vision's triage head CT solution that alerts our department of acute cases of intracranial hemorrhage. We have found the technology to be helpful as a second layer of analysis augmenting the clinical team's detection and response time for acute cases."
This is the second FDA clearance for Zebra's Multi-Modality AI Triage Solutions, which was received in May 2019 and focused on HealthPNX, an AI alert for pneumothorax (PNX), based on chest X-rays. This enabled it to be the world's first AI chest X-ray triage product, according to Zebra Medical. The company’s first FDA clearance, received in July 2018, focuses on coronary calcium scoring, which can be an early sign for coronary artery disease (CAD).
For more information: www.zebra-med.com


 March 06, 2026
March 06, 2026