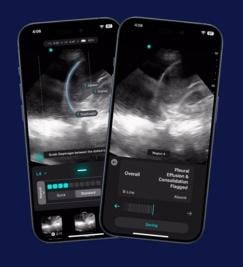
June 18, 2025 — Exo recently announced that now included on its Exo Iris is the first ever FDA 510(k) cleared AI for detecting pleural effusion and consolidation/atelectasis. These on-device real-time indicators empower clinicians with objective data to help detect lung diseases like pneumonia and tuberculosis at the bedside in seconds.
Arun Nagdev, MD, Vice President of Clinical Affairs at Exo and a leading POCUS expert, said, “This groundbreaking real-time AI provides a remarkable assist for all clinicians at the bedside, instantly and accurately detecting fluid around the lungs or areas of collapsed lung, key markers for significant infections like pneumonia or tuberculosis. This is a great support for clinicians looking to diagnose commonly presenting patient symptoms of shortness of breath, coughing, pain, and fatigue.”
Said Dornoosh Zonoobi, VP of AI at Exo, “Validated in rigorous clinical studies, these AIs enabled clinicians to detect key lung findings, with sensitivity and specificity in the ‘excellent’ range. We’re thrilled to bring these FDA-cleared firsts to all clinicians and to meaningfully impact patient diagnosis and outcomes when it matters most.”
These clearances mark not only a technical and regulatory milestone, but a meaningful expansion in how Exo is supporting frontline providers and patients when every second counts. Exo remains focused on delivering technology that enables critical decisions instantly, intelligently, and saves lives at scale.
These clearances bring to 14, the FDA-cleared AI indicators embedded in Iris. All commercialized Exo AI runs directly on Exo Iris, operates without internet, eliminates lag and enables expert-level diagnostics in any setting. Exo is establishing a new standard in fast, accurate, and scalable point-of-care imaging.
Visit Exo’s website explore these capabilities.


 March 06, 2026
March 06, 2026 









