Georgia Gov. Brian Kemp signed breast density inform bill HB62 into law May 3, 2019. The law, which becomes effective July 1, 2019, makes Georgia the 38th state to require healthcare facilities conducting mammograms to provide some form of notification about breast density to patients and referring healthcare providers.
This is a 360 degree photo view inside the neuro-interventional radiology lab at Henry Ford Hospital to show the layout ...
Gigi, a western lowland gorilla, was recently put under anesthesia at Franklin Park Zoo (Boston) so the zoo’s veterinary staff could examine her and perform further diagnostics, including a computed tomography (CT) scan, in the hopes of identifying the cause of recent health issues.
Radiology departments have many different needs and face a wide variety of challenges that can impact their departments ...
The Hong Kong Polytechnic University (PolyU) announced the development of a palm-sized 3-D ultrasound imaging system for radiation-free scoliosis assessment, dubbed Scolioscan Air. The system can bring accurate, safe and cost-efficient mass screening to schools and anywhere in the community.
The American Society of Breast Surgeons (ASBrS) has released the first screening mammography guidelines based on a patient’s individual breast cancer risk profile, with clear, specific strategies for screening management.
Bioengineers have cleared a major hurdle on the path to 3-D printing replacement organs with a breakthrough technique for bioprinting tissues. The new innovation allows scientists to create exquisitely entangled vascular networks that mimic the body's natural passageways for blood, air, lymph and other vital fluids.
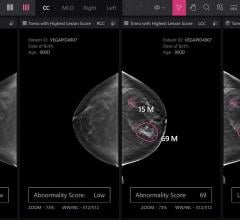
Despite decades of progress in breast imaging, one challenge continues to test even the most skilled radiologists ...
This week, cardiologists learned for the first time they have been examining black holes for decades and did not know it ...
Radiology artificial intelligence (AI) solutions provider Aidoc announced a $27 million investment, bringing its total funding to $40 million. The Series B round, led by Square Peg Capital, will be used to grow Aidoc's technology and go-to-market team.
IBA (Ion Beam Applications SA) recently announced the first Flash irradiation in an IBA proton therapy gantry treatment room at the University Medical Centre Groningen (UMCG) in The Netherlands. This novel technique has the potential to enhance the therapeutic window with a fast and powerful treatment that delivers a high dose of radiation at an ultra-high dose rate.
Bayer Radiology’s Barbara Ruhland and Thom Kinst discuss how radiology departments can address the many different ...
A new position statement from interventional radiology professional societies in the U.S. and Europe states that prostatic artery embolization (PAE) is a safe, effective and minimally invasive treatment for enlarged prostate, and should be presented as a treatment option for appropriately selected patients. The position statement was authored by the Society of Interventional Radiology (SIR), the Cardiovascular and Interventional Radiological Society of Europe (CIRSE), the Société Française de Radiologie (SFR) and the British Society of Interventional Radiology (BSIR).
May 2, 2019 — Canon Medical has installed the Aquilion One Genesis, one of the first computed tomography (CT) scanners ...
May 1, 2019 — Here is the list of the most popular content on the Imaging Technology New (ITN) magazine website from the ...
eHealth Saskatchewan plays a vital role in providing IT services to patients, health care providers, and partners such ...
International Medical Solutions (IMS) and the University of Toronto Department of Medical Imaging have signed a partnership agreement to assess the performance of diagnostic radiology residents using IMS Web Viewer within a simulated Emergency Radiology environment. The program is part of a larger study being funded by the Radiological Society of North America (RSNA) to determine the effectiveness of the ER Simulation testing program.
A comparison of three types of radiotherapy for children's brain tumors suggests a type of proton therapy called pencil beam scanning (PBS) offers the best hope of preserving cognitive functions. The study, presented at the European Society for Radiotherapy and Oncology (ESTRO) 38 conference April 26-30 in Milan, Italy, shows this new form of radiotherapy delivers the lowest doses of radiation to the temporal lobes and hippocampus, areas of the brain important in functions like memory.
International medical imaging information technology (IT) and cybersecurity company Sectra has signed a ten-year contract to install its solution for handling radiology images, Sectra PACS, across the radiology and nuclear medicine departments at Deventer Hospital in the Netherlands. The solution will support increased reading efficiency through its performance and comprehensive tool set, thereby improving patient outcomes.
April 30, 2019 — New data presented at the Society of Interventional Radiology (SIR) 2019 annual meeting showed the ...
A new document compiled by four cardiac imaging professional societies provides a resource to guide clinicians in best practices for assessing valvular regurgitation following catheter-based repair or replacement of a valve. The document, available in full here, supplements the previously published guideline Recommendations for Evaluation of Prosthetic Valves with Echocardiography and Doppler Ultrasound.
New research has shown a combination of biological markers and certain genetic changes can predict radiation sensitivity and may help identify cancer patients more likely to suffer adverse side-effects after radiation therapy. In addition, the international team of researchers in the REQUITE project found further evidence to support an earlier finding in a smaller group of breast cancer patients that the time of day when radiotherapy is given can affect whether or not patients with particular gene variants suffer from adverse side effects.
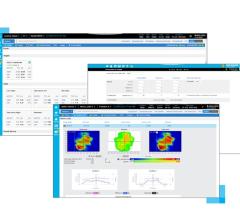
A new report by Arthur Olch, Ph.D., highlights use of specialized software at Children’s Hospital Los Angeles (CHLA) that could advance treatment accuracy of radiation oncology for pediatric cancer patients.
New medical technology offers the promise of improving patient care, as well as the potential for harm if caregivers are not sufficiently educated about its operation and use. Without a comprehensive approach, caregivers may learn how to use complex technology at the patient’s bedside, through trial and error, instruction manuals or informally from their colleagues. An article in AACN Advanced Critical Care describes how Memorial Hermann Health System in Houston developed an interdisciplinary fail-safe process to analyze and scale training for use of medical devices, with a risk assessment tool to predict the severity and frequency of potential harm to patients.


 May 06, 2019
May 06, 2019