May 2, 2019 — Radiology artificial intelligence (AI) solutions provider Aidoc announced a $27 million investment, bringing its total funding to $40 million. The Series B round, led by Square Peg Capital, will be used to grow Aidoc's technology and go-to-market team.
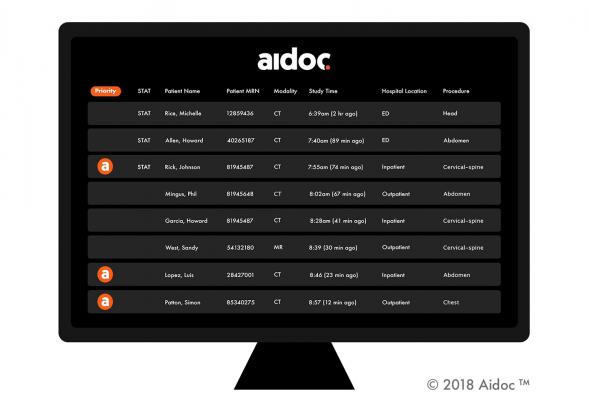
The funding comes as Aidoc announced that it has analyzed its millionth patient’s computed tomography (CT) scan in real time – the largest number of images analyzed by an AI tool, according to the company. In addition, Aidoc will be releasing its oncology line of products as well as the extension of its current suite for time-sensitive conditions to X-ray.
"Our evaluation process included numerous conversations with hospitals that are using Aidoc's solution in clinical settings, and the value they bring to patient care became evident," said Dan Krasnostein, partner at Square Peg Capital. "Aidoc is the most mature company in AI for radiology, and we believe our partnership will help fuel their triple-digit growth."
Aidoc's U.S. Food and Drug Administration (FDA)-cleared and CE-marked solutions support and enhance the impact of radiologist diagnostic power, helping them expedite patient treatment and improve quality of care. Radiologists benefit from deep learning technology that is "always-on," running behind the scenes and freeing them to focus on the diagnosis. Aidoc's solution flags the most critical, urgent cases where a faster diagnosis and treatment can be a matter of life and death.
For more information: www.aidoc.com


 February 13, 2026
February 13, 2026 









