U.S. Food and Drug Administration (FDA) Commissioner Scott Gottlieb, M.D., announced Tuesday the agency is pursuing a new framework in which to review artificial intelligence (AI)-based medical software and devices to ensure ongoing effectiveness and patient safety. The agency released a 20-page discussion paper explaining the need for a new framework, the tenets of a total product lifecycle (TPLC) approach to certification, and examples of potential real-world AI software modifications that may or may not be permitted under the proposed framework. The FDA is asking for comments and feedback from all parties to inform future decisions.
The continuing search for advantages to improve workflow has radiology departments constantly searching for new ...
In this day and age, information technology (IT) rules. It shapes the way we do business and it shapes the way we live ...
Radiology departments have many different needs and face a wide variety of challenges that can impact their departments ...
Medical imaging and visualization company Medivis announced the launch of AnatomyX, its augmented reality (AR) platform for anatomy education. Currently enabled on Microsoft's HoloLens AR technology and Magic Leap's spatial computing device, Magic Leap One, AnatomyX offers any member of a large university or medical institution an enterprise-grade learning platform for the study of human anatomy, physiology and pathology.
Medical artificial intelligence (AI) startup Lunit announced its attendance at the Society of Breast Imaging (SBI) 2019 symposium, April 4-7 in Hollywood, Fla., where it will showcase its latest product for breast cancer detection, Lunit Insight for Mammography.
Many mammography practices that use digital breast tomosynthesis (DBT) continue to use dosed digital mammography to ...
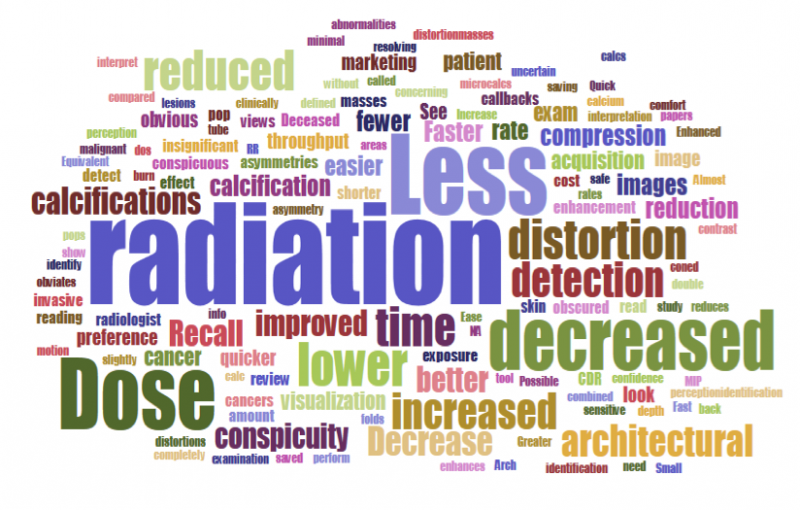
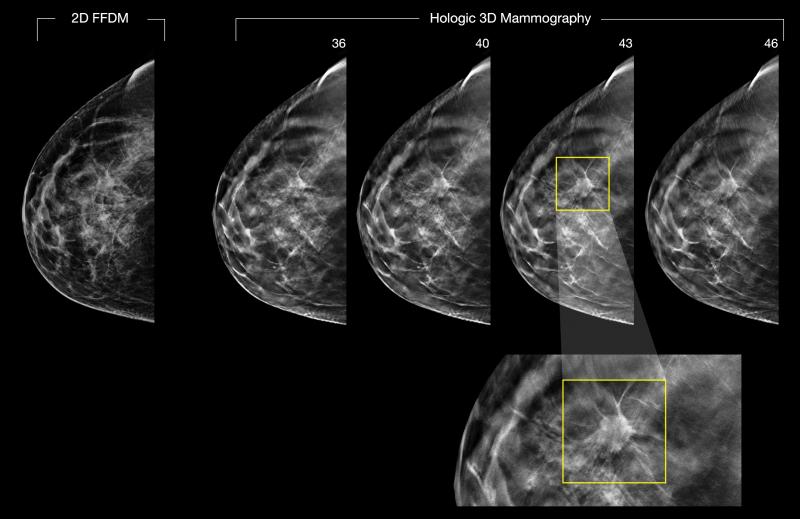
Despite decades of progress in breast imaging, one challenge continues to test even the most skilled radiologists ...
April 2, 2019 — Here is the list of the most popular content on the Imaging Technology News (ITN) magazine website from ...
At RSNA 2018, GE Healthcare formally presented Edison as the company's new applications platform, designed to speed the ...
GE Healthcare goes beyond core equipment maintenance to help clients solve some of their most important asset and ...
Bayer Radiology’s Barbara Ruhland and Thom Kinst discuss how radiology departments can address the many different ...
Paul J. Chang, M.D., FSIIM, professor and vice-chairman of radiology informatics at the University of Chicago School of ...
Dune Medical Devices has just completed the first in-man cases for Smart Biopsy, its percutaneous soft tissue biopsy device which leverages the real-time tissue characterization capability of its radiofrequency (RF) spectroscopy technology.
eHealth Saskatchewan plays a vital role in providing IT services to patients, health care providers, and partners such ...
A research team from Imperial College London believes a new software could speed up the diagnosis and treatment of patients with faulty cardiac rhythm devices in an emergency setting. The software has been able to identify the make and model of different devices, such as pacemakers and defibrillators, within seconds.
Medical imaging software company Novarad announced that it has appointed Paul Jensen as company president.
Tennis elbow, a painful chronic condition that affects up to 3 percent of U.S. adults, can be effectively treated through transcatheter arterial embolization (TAE), according to research presented at the Society of Interventional Radiology’s (SIR) 2019 Annual Scientific Meeting, March 23-28 in Austin, Texas.
GE launched a new brand that covers artificial intelligence (AI) at the Radiological Socoety of North American (RSNA) ...
The use pattern of a picture archiving and communication system (PACS) software module demonstrates that radiologists ...
GE Healthcare Centricity Clinical Archive (CCA) Analytics, shown at RSNA 2018, works directly with the vendor neutral ...
Training interventional radiologists to perform endovascular thrombectomies results in positive outcomes for patients experiencing stroke, according to a study presented at the Society of Interventional Radiology Annual Scientific Meeting, March 23-28 in Austin, Texas.1 Expanding access to this treatment provides patients timely access to this gold-standard treatment.
The U.S. Food and Drug Administration (FDA) announced new steps to modernize breast cancer screening and help empower patients with more information when they are considering important decisions regarding their breast healthcare.


 April 02, 2019
April 02, 2019