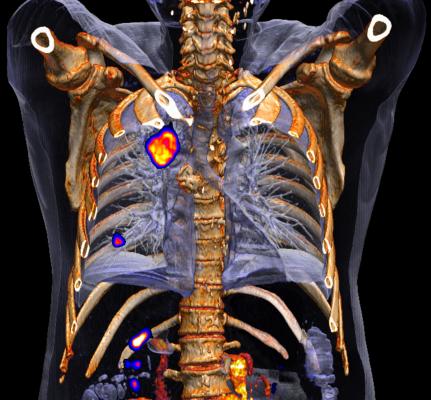
May 25, 2016 — Positron emission tomography (PET) is used commonly in the diagnosis of suspected lung cancer. Computed bioconductance (CB) id being investigated as a non-invasive way to predict whether an suspicious abnormality in the lung is benign or malignant. In a new study, researchers found that CB combined with PET before biopsy of suspicious lesions could improve diagnostic effectiveness of potentially cancerous lesions detected by computed tomography (CT) scan.
In this prospective analysis, researchers found CB results combined with PET improved the ability to distinguish malignant from benign conditions compared with PET alone.
"In China, lung cancer is the leading cause of cancer mortality and causes huge health burdens and expense. Adding CB measurement to standard PET scanning could prove to be a useful, non-invasive diagnostic tool for aiding diagnosis of patients with lung cancer" said Dawei Yang, M.D., lead author, Zhongshan Hospital Fudan University in Shanghai, China.
Study results were shared at the Chinese Thoracic Society (CHEST) World Congress 2016, April 15-17 in Shanghai.
For more information: www.journal.publications.chestnet.org


 February 13, 2026
February 13, 2026 









