March 24, 2009 - Frost & Sullivan has awarded Philips Healthcare with the 2009 North American Frost & Sullivan Product Innovation of the Year Award for its Brilliance iCT 256 slice and iCT SP 128 slice computed tomography (CT) systems.
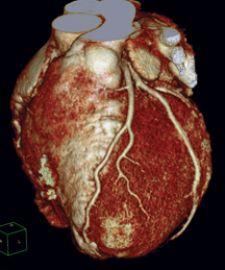
To develop the Brilliance iCT CT system, Philips has brought technology innovation to every component of the CT imaging chain. The iCT reportedly optimizes imaging speed, X-ray tube power, detector coverage and dose utility mechanisms to deliver diagnostic accuracy at lower radiation doses. The Philips Essence technology, comprised of new X-ray tubes, 8-centimeter nano-panel detectors and RapidView reconstruction, combined with other innovative technologies such as the air-bearing gantry allowing faster rotation, contribute to the Brilliance iCT's reputation as one of the most advanced CT imaging systems on the market.
"In designing the Brilliance iCT scanner, Philips has successfully overcome technical trade-offs to develop a system that is both highly capable and cost-effective," said Nadim Daher, Frost & Sullivan senior industry analyst. "The iCT was designed from the ground up to deliver the dependability and performance required in CT operations including the most mission-critical clinical environments."
The iCT also reportedly leads the way with system reliability and ease of use, two key characteristics of the Philips brand. With the iCT, Philips has effectively overcome the physical challenges of cardiac imaging such as the movement of the heart and the variability of heart rate, size and body habitus, allowing the breakthrough capability of imaging the heart within two heartbeats and with up to 80 percent dose reduction. In addition to outstanding cardiovascular imaging capabilities, the iCT constitutes an asset for the entire imaging enterprise, spanning the full spectrum of standard and advanced CT imaging services of cardiology, radiology, oncology, brain imaging, virtual colonoscopy, interventional imaging and bariatric imaging.
For more information: www.frost.com, www.medical.philips.com


 February 09, 2026
February 09, 2026 









