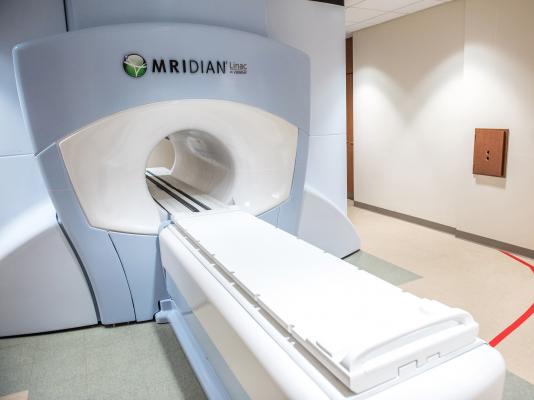
June 14, 2018 — The Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine in St. Louis has begun treating patients with Viewray's second-generation magnetic resonance (MR) image-guided radiotherapy system, the MRIdian Linac. Siteman is the first U.S. cancer center to offer cancer therapy with both MRIdian and MRIdian Linac.
Washington University physicians and researchers at Siteman played an integral role in the development, testing and clinical introduction of MRIdian in 2012. The first cancer patients worldwide to receive MR-guided radiation therapy were treated at Siteman.
Unlike conventional radiotherapy, MRIdian Linac technology uses high-definition MR soft-tissue imaging to visualize and track the detailed contours of the tumor and adjacent healthy tissues. The enhanced visibility allows clinicians to quickly and dynamically adjust the prescribed radiation dose distribution when the tumor moves during the course of treatment. The combined capabilities provide clinicians with the tools to improve targeting precision and thus deliver higher and potentially more effective radiation doses, while simultaneously minimizing incidental radiation exposure to surrounding organs.
"We know that tumors and their surrounding anatomy naturally change position from day to day — even during treatment — making it vitally important that we see where the radiation dose is being delivered in real-time," said Jeff Bradley, M.D., the S. Lee Kling Professor of Radiation Oncology at Washington University. "The clarity and detail afforded by MRI combined with this new technology allows us to target tumors with a high level of accuracy regardless of changes or motion, further enabling us to expand our treatment options."
"As an institution actively involved in advancing MR image-guided therapy since the very beginning, we are excited to remain at the forefront with the addition of this latest innovation," said Jeff Michalski, M.D., professor and vice chair of the Department of Radiation Oncology at Washington University.
For more information: www.viewray.com


 January 30, 2026
January 30, 2026 









