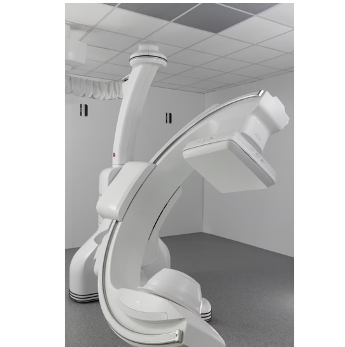
Allia Moveo (Photo: GE Healthcare)
Dec. 1, 2025 — GE HealthCare has unveiled the Allia Moveo,1 an image guiding solution designed to enhance mobility and flexibility and redefine the way clinicians deliver care in the interventional suite. This new Allia platform features a slim, compact, cable-free C-arm, allowing full movement and patient access, while aiming to deliver outstanding image quality.
To help care teams work with ease, Allia Moveo features a wide-bore C-arm designed to enable cone beam computed tomography (CBCT) that accommodates a diverse patient population, an ergonomic design and AI-powered tools intended to provide a seamless workflow, offering patients a smooth, more personalized procedure.
“As demand for minimally invasive procedures continues to increase, clinicians are navigating greater complexities and volume, and need technology that keeps pace to enhance precision, simplify workflows, and support efficient care delivery,” said Philip Rackliffe, President and CEO, Advanced Visualization Solutions (AVS) at GE HealthCare. “Allia Moveo aims to set a new standard in interventional care, reduce barriers and anticipate clinical needs, with the goal of empowering clinicians to deliver optimal patient outcomes with comfort and confidence.”
Clinicians perform nearly half of interventional procedures from working positions where they struggle with limited access to essential controls, displays, and interfaces.2 The unique design of Allia Moveo aims to ensure full access to the patient during both simple and complex procedures and includes thoughtful placement of motion controls — comfortably accessible from nearly any position — so clinicians can focus on what matters most: the procedure and the patient. These comfort-enhancing features also extend to patients, who may benefit from the wide bore and effortless table panning, which is designed to provide a smooth experience regardless of weight or body size.
In addition, Allia Moveo includes new features designed to enhance efficiency, like SmartMove, which allows the clinician to move the C-arm away from the patient whenever needed and return it to its original position at the press of a button, developed to minimize setup time and workflow interruptions. Artificial intelligence and augmented guidance tools also support advanced workflow with the goal of enabling greater precision. Like other Allia IGS systems, Allia Moveo is intended to sit at the center of the interventional ecosystem and supports multi-modality integrations across a wide range of imaging and diagnostic technologies that enable care teams to tailor their environment to the unique needs of each procedure.
“Allia Moveo is more than a new interventional system — it represents our boldest innovations yet, designed with intention and shaped by years of collaboration with interventionalists and surgeons,” said Jyoti Gera, Chief Executive Officer, CardioVascular and Interventional Solutions, AVS at GE HealthCare. “Created for and by clinicians, it is built to deliver agility, flexibility, and a reimagined workflow aimed at unlocking new levels of ease, efficiency, and precision care.”
Allia Moveo is currently FDA 510(k) pending. This product may not be available in all regions, contact your GE HealthCare sales representative for more information.
- 510(k) pending with the U.S. FDA. Not available for sale in the US. Not CE Marked.
- According to GE HealthCare sponsored blind survey conducted with 180 interventional cardiologists, interventional radiologists, and vascular surgeons in the U.S. and Europe.


 March 06, 2026
March 06, 2026 









