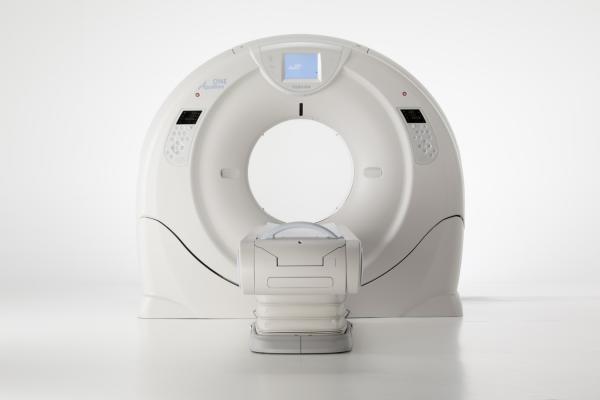
640-Slice CT Scanner Released by Toshiba
December 4, 2012 — Toshiba has unveiled a 640-slice computed tomography (CT) scanner at the 2012 annual meeting of the Radiological Society of North America (RSNA). The Aquilion One Vision Edition is equipped with a gantry rotation of 0.275 seconds, a 100 kw generator and 320 detector rows (640 unique slices) covering 16 cm in a single rotation, with the industry’s thinnest slices at 500 microns (0.5 mm). The system can accommodate larger patients with its 78 cm bore and fast rotation, including bariatric and patients with high heart rates.
Aquilion One Vision Edition also includes Toshiba’s third-generation iterative dose reconstruction software, AIDR 3-D, which incorporates significant system enhancements by reducing radiation dose compared with conventional scanning.
The system was cleared by the U.S. Food and Drug Administration (FDA) in September 2012 and currently has one install in the United States at the National Institutes of Health (NIH). Studies there on CT angiography found coronary scans could be completed in 0.0137 seconds and with less than 1 mSv of dose for an average sized patient. This less-than-a-second scan time allows for single-beat cardiac cycle scans. The 16 cm coverage area also means one scan can acquire the whole volume of the heart, so no image stitching is required.
The couch has a 660 lb capacity.
The Aquilion One Vision Edition has been installed at Fujita Health University in Japan; the National Heart, Lung, and Blood Institute (NHLBI) at National Institutes of Health in Bethesda, Md.; Radboud University Nijmegen Medical Centre in the Netherlands; Monash Medical Centre Clayton, Southern Health in Australia; Hong Kong Sanatorium & Hospital in Hong Kong; and Iwate Medical University in Japan. Future installations include University Health Network — Toronto General Hospital in Canada and Rigshospitalet in Denmark.
For more information: www.medical.toshiba.com


 February 09, 2026
February 09, 2026 









