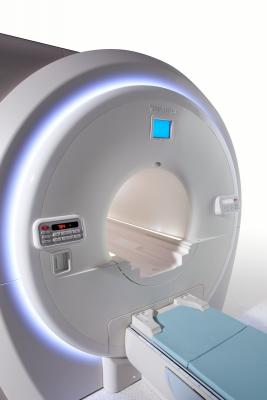
July 11, 2014 — Toshiba America Medical Systems introduced its newest M-Power software version for the Vantage Titan 1.5T magnetic resonance (MR) system and a new software version for its Vantage Titan 3T MR. Toshiba will also offer the new software versions with enhanced automated tools at no charge to existing Titan customers with previous M-Power software versions that have a current service contract with Toshiba.
The new software versions for the Titan 1.5T and 3T MR systems feature several workflow enhancements and new clinical applications:
- M-Power’s PathFinder: Improved automated tools to streamline exams and workflow efficiencies, including DirectPAS, EasyTech and InScan.
- 3-D Arterial Spin Labeling (ASL): Clinically enhanced non-contrast suite with 3-D ASL non-contrast perfusion for the whole brain.
- DelayTracker: Automation of delay times for non-contrast Fresh Blood Imaging from renal to feet vessels.
- 3-D Spectroscopy (Optional): Improved functionality to perform 3-D spectroscopy, including during prostate imaging.
- 3-D Gradient Distortion Correction: Features capabilities for 3-D correction of image distortions and is only available for the Titan 3T MR.
“Toshiba puts customers’ business needs first when developing software advancements so that healthcare providers can deliver the best patient experience with the latest technology available,” said Beverly Plost, director, MR Business Unit, Toshiba. “The M-Power software for Toshiba’s Titan 1.5T and Titan 3T MR includes enhancements to a comprehensive suite of innovative applications for faster and easier exams.”
For more information: www.medical.toshiba.com


 January 28, 2026
January 28, 2026 









