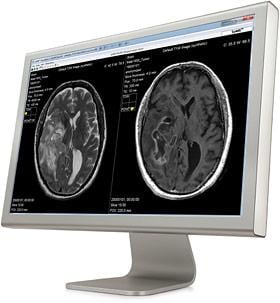
September 2, 2015 — Linköping University Hospital in Sweden has installed SyMRI Neuro from SyntheticMR in order to improve the follow-up of patients with multiple sclerosis (MS). SyMRI enables objective follow-up of brain atrophy through automatic calculation of brain parenchymal fraction (BPF). After an initial pilot project, the aim is to take SyMRI into clinical use in Region Östergötland during 2016.
Magnetic resonance imaging (MRI) is especially good at visualizing soft tissue in the body and is used in the investigation and monitoring of MS. With current methods, however, physicians are for the most part forced to make subjective judgments of the images, which can make it difficult to obtain a good and comparable measure of disease development.
“With SyMRI we can get an automatic measurement of brain atrophy, which means that we can now quantify the neurodegeneration that occurs in connection with MS. This allows for a better follow-up for our patients”, said Patrik Fägerstam, M.D., neuroradiologist at Linköping University Hospital.
SyMRI Neuro will be installed in the hospital’s picture archiving and communication system (PACS) from Sectra.
For more information: www.symri.com


 February 13, 2026
February 13, 2026 









