Getty Images
March 23, 2022 — With today’s U.S. Food and Drug Administration (FDA) approval of 177Lu-PSMA-617—a radiopharmaceutical therapy to treat metastatic castration-resistant prostate cancer (mCRPC)—the Society of Nuclear Medicine and Molecular Imaging (SNMMI) celebrates one of the greatest success stories in nuclear medicine history. The therapy, developed after years of extensive research, has been shown to reduce the risk of death by 38 percent and reduce the risk of progression by 60 percent in mCRPC patients treated with 177Lu-PSMA-617.
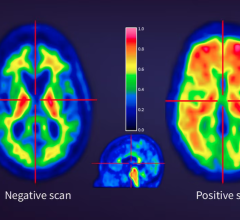
The new therapy depends on the use of PET scans to identify and treat patients whose metastatic prostate cancers express PSMA (prostate specific membrane antigen). “We are delighted by the FDA approval of this transformational, innovative therapy for men with advanced metastatic castrate resistant prostate cancer,” said SNMMI president Richard L. Wahl, MD, FACNM. “We are proud of the society members who contributed substantially to this new theranostic paradigm, as well as all of the authors who published articles on this therapy in The Journal of Nuclear Medicine.”
The FDA granted Priority Review for 177Lu-PSMA-617 in September 2021 based on positive data from the multi-center Phase III VISION study, which measured overall survival and progression-free survival in mCRPC patients whose disease progressed despite treatment.
This work builds on the success of prior radiopharmaceutical therapies such as 177Lu-DOTATATE, which has provided a significant clinical benefit to patients with neuroendocrine tumors. SNMMI plans to provide guidance and support to physicians and patients as 177Lu-PSMA-617 becomes readily available. The society has updated its appropriate use criteria for PSMA PET imaging to include an indication of “Evaluation of eligibility for patients being considered for PSMA-targeted radioligand therapy,” which was scored as ‘appropriate’ given the availability of a PSMA targeted therapy. Additionally, resources will be developed to educate mCRPC patients about the new therapy.
“The FDA approval of 177Lu-PSMA-617 is a testament to what nuclear medicine innovators, working closely with clinical colleagues in the prostate cancer care domains, can accomplish with their unique combination of expertise in basic biology, radiochemistry, physics, and instrumentation,” noted Wahl. “The development of radiopharmaceutical therapies is advancing rapidly, and we fully expect there will be more to come as they can be so effective and beneficial for patients fighting cancer.”
Prostate cancer is the second most common cancer in men. More than 268,000 cases of prostate cancer and approximately 34,500 deaths are projected in the United States in 2022. While the five-year survival rate of localized prostate cancer is nearly 100 percent, for mCRPC patients it is only 30 percent.
177Lu-PSMA-617 is manufactured by Novartis.
For more information: www.snmmi.org
Related prostate cancer content:
PSMA PET Validates EAU Classification System to Determine Risk of Prostate Cancer Recurrence
VIDEO: MRI-Linac and PSMA PET Imaging Technologies Aids Therapy at GenesisCare
FDA Approves First Commercially Available PSMA PET Imaging Agent for Prostate Cancer
PSMA-Targeted Radiotracer Pinpoints Metastatic Prostate Cancer Across Anatomic Regions
Metastatic Prostate Cancer on the Rise Since Decrease in Cancer Screenings
Rational Surgical Solutions’ mCRPC Master Now Offered as Free Download
A Look Ahead in Targeted Radionuclide Therapy


 February 03, 2026
February 03, 2026