January 24, 2022 — New research has confirmed the accuracy of the novel European Association of Urology (EAU) risk classification system that groups prostate cancer patients based on their risk of recurrence. Prostate-specific membrane antigen (PSMA) PET imaging of men with prostate cancer validated the EAU groupings and provided insights that could further refine risk assessment for patients. This study was published in the January issue of The Journal of Nuclear Medicine.
The diagnostic workup of prostate cancer has changed rapidly over the past few years. Recently, the EAU introduced a clinical system separating patients with rising PSA values after first-line therapy (prostate surgery or radiation) into groups of those with high risk and those with low risk for development of metastases. Shortly after this, the U.S. Food and Drug Administration approved 68Ga-PSMA-11 as the first PET drug to target the PSMA for men with prostate cancer.
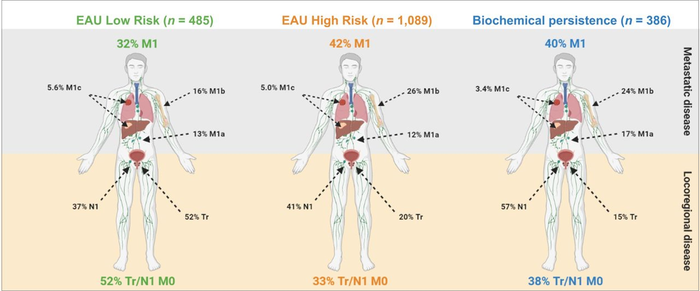
“Given the growing availability of PSMA-directed PET imaging, our study sought to assess disease in patients based on the EAU classifications while using PSMA PET to identify subgroups of patients, such as those with undetectable, locoregional or distant metastatic disease,” said Justin Ferdinandus, MD, nuclear medicine physician at University Hospital in Essen, Germany.
The multicenter, international study analyzed PSMA PET scans of nearly 2,000 patients with prostate cancer and rising PSA levels. Patterns of disease spread on PSMA PET imaging were used to classify prostate cancer patients into both low- and high-risk groups. High-risk groups were found to have higher rates of metastatic disease on PSMA PET compared to low-risk groups. However, PSMA PET also found metastatic disease in low-risk and no disease in high-risk patients.
“Our study underscores the utility of the EAU risk groups to determine risk of metastasis in biochemically recurrent prostate cancer. But not every high-risk patient has metastases and not every low-risk patient has locoregional or no disease,” said Wolfgang Fendler, MD, nuclear medicine physician at University Hospital in Essen.
He continued, “The ultimate aim of imaging is to provide the right treatment for each patient. As evidenced in this research, the accuracy of PSMA PET is essential to improve stratification and potentially outcomes both in low-risk and high-risk settings.”
For more information: www.snmmi.org
Related prostate cancer content:
VIDEO: MRI-Linac and PSMA PET Imaging Technologies Aids Therapy at GenesisCare
FDA Approves First Commercially Available PSMA PET Imaging Agent for Prostate Cancer
PSMA-Targeted Radiotracer Pinpoints Metastatic Prostate Cancer Across Anatomic Regions


 February 13, 2026
February 13, 2026 









