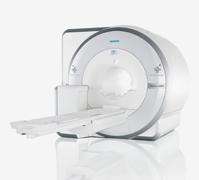
December 1, 2010 – One of the world's first whole-body MR/PET systems was introduced at the RSNA 2010 meeting. The Biograph mMR, by Siemens, combines an magnetic resonance (MR) imaging scanner with an integrated positron emission tomography (PET) detection system to simultaneously capture both MR and PET data.
The 3-Tesla hybrid system has been installed at the University Hospital “Klinikum rechts der Isar” of the Munich Technical University in Germany.
“Together with our partner Siemens, we are entering a new dimension in diagnostic imaging today,” said Markus Schwaiger, professor, director of the Clinic for Nuclear Medicine at the University Hospital. “We’ve initiated clinical use testing of Biograph mMR in an effort to diagnose diseases at a very early stage to see the progression of disease and to use that information to develop a therapy plan precisely focused on the respective patient. Furthermore, we plan to use the system for cancer follow-up in the long run, by reducing radiation exposure by the use of the system.“
With the simultaneous acquisition of MR/PET data, this system is designed to provide new opportunities for imaging. While MR provides morphological and functional details in human tissue, PET goes further to investigate the human body at the level of cellular activity and metabolism. The system has the potential to be a particularly valuable tool for identifying neurological, oncological and cardiac conditions of disease and in supporting the planning of appropriate therapies. Since MRI does not emit ionizing radiation, it may provide an added benefit with lower-dose imaging. It also opens new opportunities for research, such as the development of new biomarkers or new therapeutic approaches.
The Biograph mMR is designed to simultaneously acquire morphology, function and metabolism for the entire body.
Initial research suggests that with this system, molecular MR can scan the entire body in as little as 30 minutes for the combined exams, compared to one hour or more for sequential MR and PET examinations.
Until now, it was nearly impossible to integrate MR and PET technologies. The conventional PET detectors, which use photomultiplier tubes, could not be used in the strong magnetic field generated by an MR system. Integration was further limited by the lack of space inside the MR device.
The system has not been reviewed by the U.S. Food and Drug Administration (FDA). Siemens is seeking European CE mark approval for the system, which it hope to gain in 2011.
Philips Healthcare also unveiled its PET/MR scanner at RSNA 2010. The company said it has already filed for FDA 510(k) review and hopes the system might gain market clearance in 2011.
For more information: www.siemens.com/healthcare


 February 13, 2026
February 13, 2026 









