November 30, 2020 — Resoundant Inc., announced that it has entered into a strategic partnership with United Imaging Healthcare to begin offering advanced magnetic resonance elastography (MRE) on leading United Imaging MRI systems.
United Imaging’s mission encompasses a dedication to the latest innovations and ensuring their accessibility worldwide. The company’s unique “all in” model features the highest priority applications needed to meet day to day workflows. The inclusion of MRE as a core product will exemplify this standard, as it has become an essential clinical tool for non-invasively assessing liver fibrosis around the world.
“We are thrilled to partner with United Imaging to deliver MRE on their cutting-edge MR solutions,” said Kathy Anderson, JD, Chief Operating Officer of Resoundant. “This partnership will further our overarching mission: to radically advance patient care through exploration, development, and dissemination of high–impact applications of MR Elastography.”
Reflecting a dedication to breakthrough technologies, the two companies have also agreed to work towards introduction of the next generation of MR Elastography, called 3D-MRE, as a standard product for 1.5T and 3.0T systems.
“If we look at the adoption of MRE so far, many countries in the Asian-Pacific region have done a tremendous job in providing access to MRE,” said Michael Kalutkiewicz, Vice President of Global Policy and Communications of Resoundant. “Our partnership with United Imaging will ensure even more patients have access to advanced MRE.”
The two companies are currently in final stages of testing and implementation with a goal for delivery in 2021.
About MR Imaging for Chronic Liver Disease
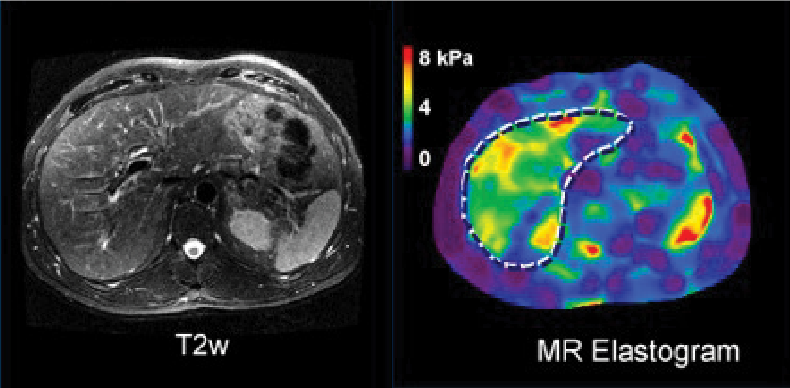
Quantitative imaging technologies offer powerful capabilities to address the global problem of chronic liver disease. For many patients, MRE can serve as a reliable, less expensive, and safer alternative to biopsy to diagnose and stage liver fibrosis.
The technical advantages of MRE for assessing fibrosis and PDFF for assessing liver fat have been well-validated in dozens of studies against paired biopsies, with high degrees of inter-reader agreement and repeatability. Both biomarkers have also been standardized across various vendor platforms and field strengths and utilize the same quantitative cutoffs regardless of the underlying etiology (viral hepatitis, fatty liver disease, etc.) – making them ideal for clinical research. Neither is significantly affected by common co-morbidities that can cause failure in complementary ultrasound-based techniques, such as obesity.
About MRE
MR Elastography was invented at Mayo Clinic. It is widely available to clinicians at over 1,600 locations across the globe and is the only MRI technology that has been validated for staging liver fibrosis. Clinicians and patients can find U.S. locations at MRE:connect. The role of MRE has been increasingly recognized in multidisciplinary clinical guidelines for routine liver fibrosis assessment, particularly in suspected cases of non-alcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH). The American College of Radiology issued Appropriateness Criteria that identify MRE as the most accurate and applicable noninvasive liver fibrosis exam. MRE is reimbursed via a Category I CPT code (76391) and is covered by numerous public and private insurance plans.
For more information: www.resoundant.com


 February 13, 2026
February 13, 2026 









