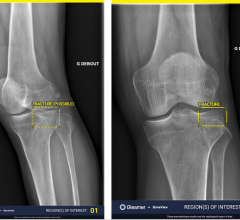
January 9, 2014 — Viztek, a provider of digital software and hardware diagnostic imaging solutions, announced that the Platinum Dynamic Fluoroscopy System has received U.S. Food and Drug Administration (FDA) 510(k) clearance. The system offers advanced
digital radiography (DR) technology, enabling healthcare facilities to manage both digital
X-ray as well as a variety of
fluoroscopy subtypes all in one exam room.
"Due to space constraints in many facilities and the need for quick turnaround time, the Platinum Dynamic Fluoroscopy System was designed to provide a streamlined workflow and saves valuable physical space," said Bruce Ashby, general manager of DR, Viztek. "The system's high image quality and fast patient throughput, for both radiography and fluoroscopy scans, makes it the ideal replacement solution."
Because the dual-purpose Platinum Dynamic System enables both fluoroscopy and DR to be performed in one room, it saves facilities space, increases workflow and lowers the total cost of ownership. It provides for a range of fluoroscopy exams including gastrointestinal, pediatrics, injections, tomography, arthrography, urogenital and digital subtraction angiography (DSA). When used in static mode, the system also delivers throughput of high-quality DR images in as little as five seconds. The Platinum System features a 17 x 17-in. panel that provides a field view large enough to eliminate the need for panel rotation.
The compact design of the Platinum Dynamic System adds to patient comfort while also streamlining workflow and eliminating the need for additional real estate within the facility. Key benefits include:
- Ability to access patients from all around the table — a benefit for the elderly or those that are difficult to position.
- Compared to other systems, Viztek's Dynamic System has a source image distance (SID) of 40 to 72 inches.
- Capability of rotating 90/90 degrees.
- Ability to support patients up to 584 lbs.
- Capability to lower to an 18.9-in. horizontal tabletop height to offer 79 in. of patient coverage, which minimizes patient repositioning and providing facilities with a flexible solution for patient convenience.
Apelem, a subsidiary of the DMS Group is the manufacturer. Viztek is the distributor of the Dynamic System in the United States.



 February 10, 2026
February 10, 2026