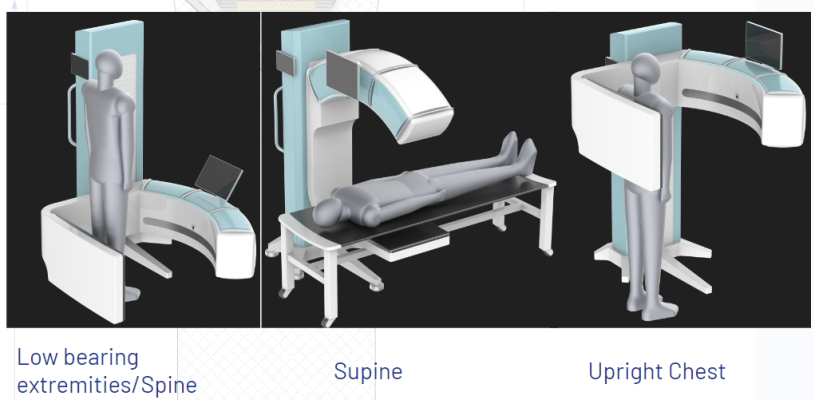
February 26, 2024 — AIxSCAN, Inc. began clinical trials in late 2023. The AIxSCAN, Inc. team is very pleased with the early ARC60 imaging results, both in terms of depiction of details and consistency of imaging quality.
AIxSCAN, Inc. received additional seed funding in early September 2023 to support clinical trials and engineering projects.
UC San Diego Health is the first site for the clinical trials to be conducted.
Between late 2023 and early 2024, AIxSCAN, Inc. plans to produce over 50 lung disease patients scans in the U.S. and up to 1,000 total lung disease patients scans within 2 years. AIxSCAN, Inc. will use some of the data to support its 510k submission to the FDA in 2024.
AIxSCAN, Inc. is also in final discussions to partner with three more institutions as soon as additional ARC60 units come out of production.
Norbert Pelc, Sc.D., Professor of Radiology, Emeritus at Stanford University and AIxSCAN, Inc. Advisory Board member, is also very impressed with the ARC60 imaging platform capabilities. He said: "This innovative technology has the potential to change the way we diagnose a range of medical conditions. The AIxSCAN tomosynthesis X-ray scanner does not completely avoid the superimposition limitations of radiography, but the early results from the prototype system are very encouraging and suggest the technology can play a significant role. I believe that AIxSCAN's tomosynthesis X-ray scanner has a very good chance of becoming a valuable tool for the medical community. This technology has the potential to improve patient care and save lives."
For more information: www.aixscan.com


 March 06, 2026
March 06, 2026 









