CAAS MRV is part of Pie Medical Imaging's suite of imaging solutions for quantitative cardiovascular diagnosis and treatment planning.
September 1, 2015 — Based on its recent analysis of the cardiovascular image management market, Frost & Sullivan recognizes Pie Medical Imaging (PMI) with the 2015 European Frost & Sullivan Award for Technology Leadership. The Netherlands-based PMI has designed innovative and easy-to-use imaging solutions — its CAAS and 3mensio product lines — which deliver precise and reproducible quantitative results for cardiovascular diagnosis and treatment planning. By improving the quality of cardiovascular diagnostics and interventions, the software is effectually facilitating next-generation research on the efficacy of novel intervention methods.
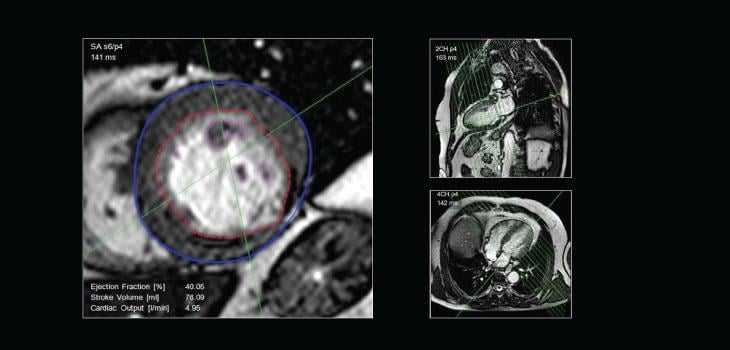
Among existing imaging modalities, cardiovascular magnetic resonance imaging (CMR) plays a role of growing importance in the assessment of patients with heart failure (HF). Currently, it is difficult to study the myocardial perfusion status in HF due to left ventricular (LV) remodeling and wall thinning, coexistent scars and respiratory artefacts. There is an urgent need for state-of-the-art imaging modalities with software algorithms to diagnose cardiovascular diseases accurately and at an early stage. In response to this need, PMI developed CAAS MRV, a sophisticated software that provides quantitative analyses of cardiovascular magnetic resonance images.
Left and right ventricular analysis from CMR datasets and blood flow measurements from velocity-encoded MR will assist the medical specialist in selecting the best treatment options. For flow analysis, PMI offers CAAS MR Flow and recently released its highly innovative CAAS MR 4D Flow to complete its CMR analysis portfolio.
During interventional procedures, as performed in the catheterization laboratory under X-ray guidance, other analysis tools are required. "Unlike visual estimations of coronary artery dimensions, CAAS QCA automatically determines stenosis severity, which refines the visual estimate and provides objective and reproducible measurements of several important features of the coronary anatomy," said Frost & Sullivan Industry Analyst Darshana De. "To develop the CAAS platform further, PMI has more than 60 full-time R&D resources dedicated to developing and optimizing the advanced visualization and analysis software."
PMI is the market leader in cardiovascular analysis software for cardiology and radiology. Its CAAS and 3mensio brands offer a broad range of products for the analysis of X-ray, MRI, computed tomography (CT), optical coherence tomography (OCT) and intravascular ultrasound (IVUS) images. Its unique products provide:
- Aortic regurgitation analysis based on X-ray angiography for use in aortic valve replacement procedures (CAAS A-valve);
- Vessel analysis from MR datasets for research, including bifurcation analysis, vessel wall determination and plaque composition analysis (CAAS MRA);
- Pre-op planning software for aortic valve, mitral valve and left atrial appendage interventions based on CT imaging (3mensio Structural Heart);
- Abdominal aneurysm repair (EVAR) analysis software based on CT imaging (3mensio Vascular); and
- 3-D coronary reconstruction and analysis from two X-ray projections (CAAS QCA3D).
PMI has close ties with research institutions in Europe, the United States, Japan and Australia. These partnerships include evaluation studies and co-development of products and solutions. It also works with core labs such as CRF, New York and Cardialysis in Rotterdam to study the efficacy of new treatment methods.
"PMI's CAAS Workstation is a stand-alone modular software product for the viewing and quantification of X-ray angiographic images and runs on a standard personal computer," noted De. "Its quantitative analyses results are based on semi-automatic contour detection forms and can be exported in various formats across different consoles, independent of the type of vendor acquisition equipment."
The CAAS Workstation offers segmentation of different cardiovascular structures, 3-D reconstruction of vessel segments based on two X-ray projections, as well as measurement and reporting tools. It aids in calculating the dimensions of cardiovascular structures, quantifying stenosis in coronary and peripheral vessels, the motion quantification of left and right ventricular walls, as well as stent visualization and dimension measurement.
For more information: www.piemedicalimaging.com


 February 13, 2026
February 13, 2026 









