
December 15, 2014 — A new lung screening solution from Philips offers healthcare providers a faster and more definitive pathway to lung cancer detection and treatment. Making its debut at the 2014 Radiological Society of North America (RSNA) annual meeting in Chicago, the solution is comprised of products and services that enable healthcare providers to implement and manage a comprehensive computed tomography (CT) lung screening program which tracks and guides patients across the health continuum.
Philips' solution can be used with most low-dose CT scanners and consists of an integrated portfolio of products and services that together offers healthcare providers a complete program for early lung cancer detection and ability to easily assess individual patient status as well as overall program key performance indicators.
"Lung cancer has a significant mortality rate in the U.S., and given the large number of heavy smokers in the aging baby boomer population, we can expect to see that number rise in the coming years," said Andrea McKee, M.D., chair of the radiation oncology department at Lahey Hospital and Medical Center in Burlington, Mass.
The Philips solution consists of several elements:
- Services Marketing – Consultative services and marketing support enables health systems to reach high-risk patients and their primary care physicians (PCPs), provide connections to patient advocacy and support organizations, information sources and access to materials for marketing their lung cancer screening services.
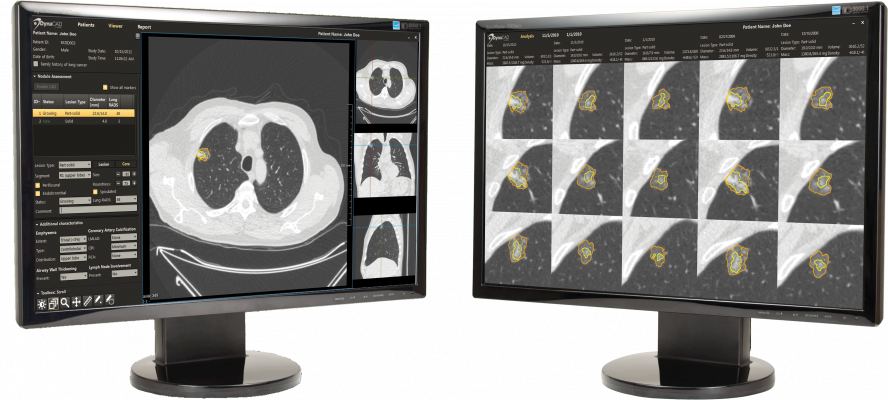
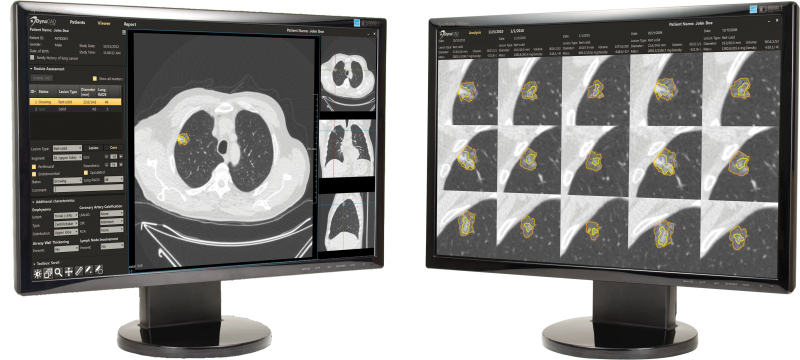
- Patient Management – "Control center" software tools allow providers to follow a set of customized clinical protocols and establish interfaces with other hospital systems (health information and radiology information systems [HIS/RIS], electronic medical records [EMR] and picture archive and communication systems [PACS]), providing clinical teams with a 360-degree view of patients. Customizable workflow tools also send timely and accurate notifications, and track participant history and touch points.
- Radiology Workflow – CT lung image review and reporting offers specialized radiology software tools which facilitate review and reporting of serial CT lung exams to identify and easily follow areas of concern.
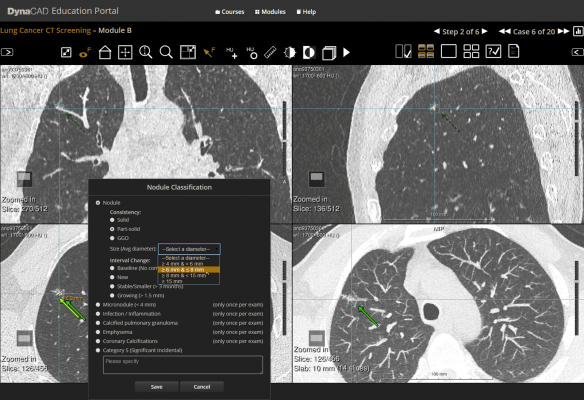
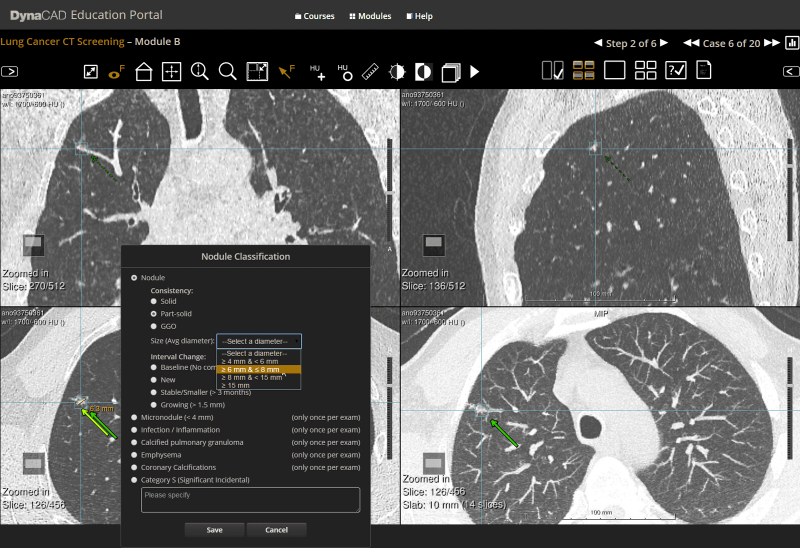
- Physician Education Resources – Comprehensive online education portal to measure and report radiologist performance against a wide range of clinical cases, as well as structured education courses and other reference materials.
The Philips lung screening and management system will be available in North America in the first half of 2015.
For more information: www.philips.com/healthcare


 February 09, 2026
February 09, 2026 









