March 3, 2016 — Participating dementia specialists may now enroll patients in the Imaging Dementia—Evidence for Amyloid Scanning (IDEAS) Study at IDEAS-Study.org. Primary care and other doctors not taking part in the IDEAS Study are encouraged to refer eligible patients to participating physicians.
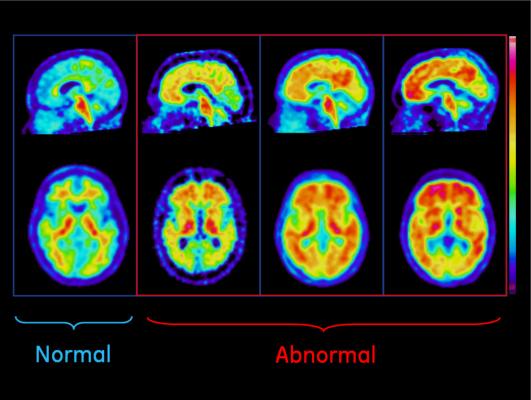
The IDEAS Study will follow more than 18,000 Medicare beneficiaries to determine the clinical value of a brain positron emission tomography (PET) scan to detect the hallmark brain amyloid accumulation of Alzheimer's disease in diagnosing and managing treatment of patients age 65 and older with mild cognitive impairment (MCI) or dementia of uncertain cause. Information from this scan can help exclude underlying Alzheimer's disease, and may help guide patient management.
"We are excited that approved physicians may begin registering patients for the IDEAS Study. We anticipate that results from the study will greatly inform future treatment and coverage decisions that can benefit countless Americans and others around the world," said Gil D. Rabinovici, M.D., IDEAS Study principal investigator and associate professor of neurology at the University of California, San Francisco.
Board-certified neurologists, psychiatrists and geriatric medicine physicians interested in enrolling eligible patients in the IDEAS Study, along with PET facilities that would like to join the study as imaging sites, may apply for participation online using the register/login portal. Participating physicians may enroll eligible patients using the same portal. Background on the study, requirements for participation and a set of frequently asked questions are available on the IDEAS website.
"The IDEAS Study will provide the evidence needed to demonstrate the utility of amyloid PET imaging in a clinical setting and for future decision making about insurance coverage for what we believe to be an important diagnostic tool," said Maria Carrillo, Ph.D., Alzheimer's Association chief science officer and IDEAS Study co-chair. "A swift and accurate diagnosis has a huge impact on access to Alzheimer's treatments, eligibility for research trials, plus much-needed support and information services."
The IDEAS Study will not directly recruit patients. Participants must instead be referred into the study by dementia specialists. Dementia specialists will enroll patients whose cases meet the study enrollment criteria and refer them for an amyloid PET scan. Amyloid PET scans will be performed and interpreted by a nuclear medicine physician or radiologist, with results provided to the ordering physician for disclosure to the patient and to support further diagnostic decisions. Scan results and diagnosis will be captured for the study.
Participating PET facilities and interpreting physicians will be reimbursed for the scans under the Centers for Medicare & Medicaid Services (CMS) Coverage with Evidence Development (CED) policy that requires research study participation as a condition of Medicare payment. This is currently the only way a brain amyloid PET scan will be covered for Medicare beneficiaries.
The IDEAS Study is led by the Alzheimer's Association and managed by the American College of Radiology (ACR) and American College of Radiology Imaging Network (ACRIN).
For more information: www.ideas-study.org


 February 13, 2026
February 13, 2026 









