July 6, 2015 - The Dr. Samadi Prostate Cancer Center in New York City is now offering a full suite of new genetic testing methods for diagnosing prostate cancer. The new methods are intended for men with an elevated prostate specific antigen (PSA) level or who have had a biopsy.
The center is offering a range of genetic tests for assessing the risk and optimizing the diagnosis of prostate cancer, both pre-biopsy and post-biopsy.
The difference is the role genetic testing plays throughout the entire process, from diagnosis to recurrence. If a man's PSA is elevated, the 4K Score, PcA3, and prostate biopsy can further indicate activity around the prostate as well as support whether to get a biopsy or not. If a biopsy returns positive, the next step is directing the right prostate cancer treatment for each patient. Other genetic testing methods such as Prolaris and OncotypeDx, combined with testing for hereditary PCa and genes like BRCA, will give more information regarding the risk factors, growth rate, aggressiveness and risk of morbidity of the cancer.
The Dr. Samadi Prostate Cancer Center will offer the following genetic tests:
Post-PSA Diagnostic Testing
4K Score: This test measures four prostate-specific kallikreins in the blood: Total PSA, FREE PSA, Intact PSA and Human Kallikrein 2 (hK2). Results are combined with patient age, digital rectal exam (nodule, no nodule) and prior negative biopsy results (yes, no). The tests then provides a percentage probability on a scale of 1-95 for the patient having high-grade prostate cancer. The 4K Score is designed specifically to reduce the number of unnecessary negative biopsies that detect low-grade cancer. This means not all men who have an elevated PSA will require a biopsy.
PCA3 Score: This is a simple urine sample collected following a digital rectal exam for the determination of the PCA3 score. Specific for prostate cancer, and, unlike the PSA, this test is not affected by prostate enlargement or other non-cancerous prostate conditions. In combination with PSA and DRE results, the PCA3 score provides useful information to help decide if a biopsy is needed, or can be delayed. It is much more specific in giving additional information about the aggressiveness of the cancer if the patient has a positive biopsy.
Post-Biopsy Genetic Testing
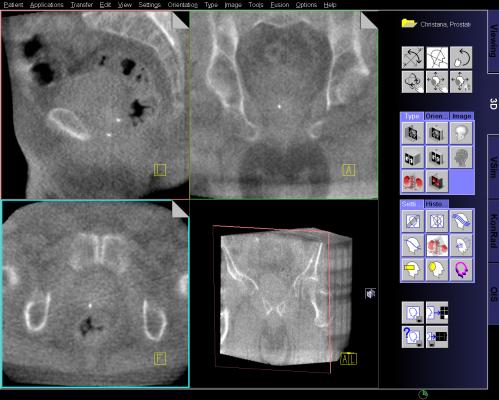
MRI Guided Biopsy: Dr. Samadi's Prostate Cancer Center uses the Uronav Fusion Biopsy System for magnetic resonance imaging (MRI)-guided biopsy. An MRI-ultrasound fusion biopsy involves taking an MRI and then fusing the data with real-time ultrasound images for guidance on biopsy procedures. The UroNav System combines electromagnetic tracking and navigation with an onboard computer and a real-time imaging interface in one mobile workstation. The MR/ultrasound fusion aligns and registers prior diagnostic MR images with real-time ultrasound images.
Oncotype DX: The Oncotype DX Genomic Prostate Score is a biopsy-based genetic test that can be combined with other measures to predict the aggressiveness of prostate cancer. The test applies advanced genomic science to reveal the unique biology of a tumor in order to optimize cancer treatment decisions for each individual patient. The test is a multi-gene RT-PCR expression analysis developed to work in combination with prostate needle biopsies. It measures the expression of 12 cancer-related genes representing four biological pathways and 5 reference genes, which are then combined to calculate the Genomic Prostate Score (GPX). This biopsy-based score has been clinically validated as a predictor of aggressive prostate cancer.
Prolaris & Genomic Prostate Score: The purpose of this score is to distinguish between aggressive cancers that need treatment and those that are slow growing and may need active surveillance. The Prolaris Score is a measure of how fast a prostate cancer tumor is growing after a biopsy has indicated its presence in the prostate gland. Biopsy tissue samples are used to determine a patient's personal Prolaris Score. It measures how fast cancer cells in the tumor are dividing. Measuring a 46-gene expression signature, Prolaris also includes cell cycle progression genes selected based upon correlation with prostate tumor cell proliferation.
ConfirmMDx: A genetic test rooted in the field of epigenetics, this test identifies key changes in gene activity for a negative biopsy or results showing high-grade PIN or ASAP. ConfirmMDx was developed to help reduce unnecessary repeat biopsies through its support of the negative predictive value (NPV). Clinical trials showed this test to be the most significant independent predictor for prostate cancer detection on repeat biopsy.
"Now that we've moved from ultrasound to MRI, in combination with these genetic tests, we may actually be to able crack the code and diagnose aggressive prostate cancer earlier. And of course, this leads to the right treatment path for each patient," said Samadi.
For more information: www.prostatecancer911.com


 March 09, 2026
March 09, 2026 









