January 4, 2018 — Mevion Medical Systems has received U.S. Food and Drug Administration (FDA) 510(k) clearance for the Mevion S250i Proton Therapy System including Hyperscan pencil beam scanning (PBS) technology. Hyperscan PBS introduces novel energy layer switching and automated collimation systems. These advantages enable the S250i system to deliver faster, sharper and more robust PBS proton radiation treatments.
The S250i with Hyperscan PBS has now received FDA and CE mark clearance.
The Mevion S250i system is a compact proton therapy system capable of delivering conformal radiation therapy treatments using Hyperscan pencil beam scanning technology. The design of Hyperscan PBS technology overcomes clinical challenges that were previously faced by first-generation PBS systems.
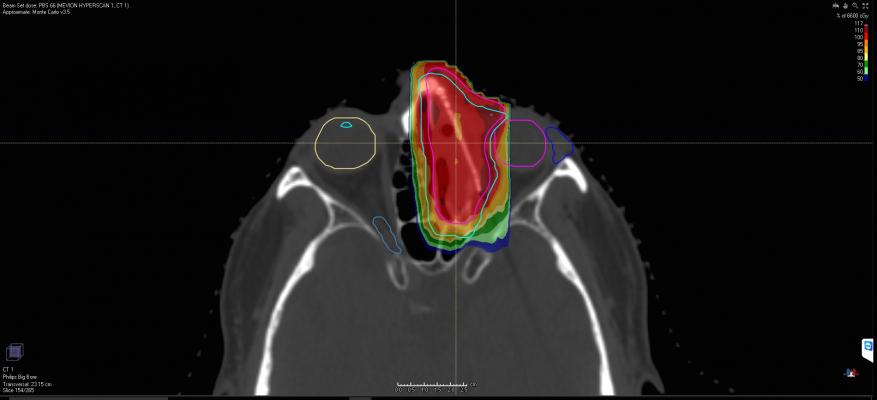
Pencil beam scanning systems shape the delivered radiation dose by “painting” tumors spot-by-spot and layer-by-layer with sub-atomic particles. Prior to Hyperscan PBS, scanning proton systems struggled with delivery speed, according to Mevion. Long delivery times can undermine the high precision of the treatment due to the target tumor shifting under normal organ motion such as breathing.
Hyperscan PBS uses a compact beam delivery path reducing delivery times to less than 5 seconds for some fields. This “hyper-fast” treatment delivery reduces treatment errors due to the sensitivity to motion that current PBS technologies face when treating tumors affected by organ motion.
In addition, Hyperscan PBS utilizes the Adaptive Aperture proton multi-leaf collimator (pMLC). This technology uses a robotically controlled collimation system, capable of trimming the edges of the beam at every layer of delivery. This capability delivers up to a three times sharper drop off in radiation at the delivery field edge. This improves sparing of healthy tissue and limits unnecessary radiation to sensitive locations.
MedStar Georgetown University Hospital in Washington, D.C. will be the first hospital in the world to offer this latest generation of Hyperscan PBS once final onsite testing is completed in January.
“We are excited to be not only the first and only proton therapy system in the Washington, D.C. area, but also the first in the world to offer these advanced proton therapy treatment capabilities to our patients and community. Currently, patients who seek proton therapy need to leave the metropolitan D.C. area, which can be a significant burden on families,” said Brian T. Collins, M.D., clinical director of MedStar Georgetown Proton Therapy Center. “We will now be delivering advanced proton therapy, fully integrated into our broad set of comprehensive cancer care offerings. This is critical to the patients we serve.”
The Mevion S250i system is based on Mevion’s high-efficiency, low-financial-risk S250 Series platform. The core technology of the S250 Series is what the company calls the world’s only gantry mounted superconducting synchrocyclotron.
For more information: www.mevion.com
Related Content
VIDEO: Clinical Considerations for Proton Therapy


 January 30, 2026
January 30, 2026 









