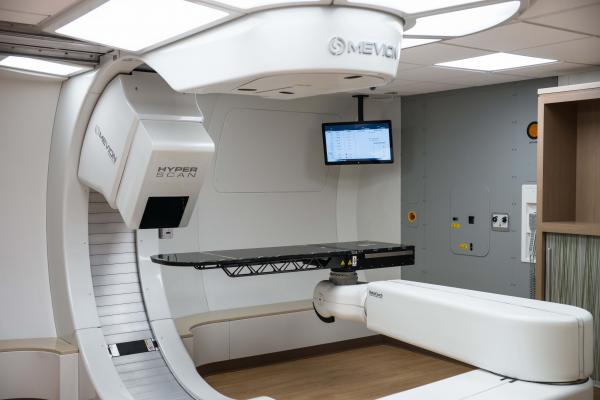
November 21, 2017 — Mevion Medical Systems announced the Mevion S250i Proton Therapy System has achieved CE Marking clearance. CE Marking permits clinical use of the system within the European Union and any country that recognizes the CE Mark. The system includes Hyperscan technology, the next generation of pencil beam scanning (PBS) for faster, sharper and more robust proton treatments.
European CE Marking is the first regulatory clearance for the Mevion S250i. Mevion also submitted for U.S. Food and Drug Administration (FDA) 510(k) clearance in September.
The first European S250i proton therapy system installation will be completed in 2018 at the Zuid-Oost Nederland Protonen Therapie Centrum (ZON PTC) at the Maastro Clinic in Maastricht, the Netherlands. The system will be the first of its kind in Europe. The Hyperscan PBS technology incorporates the fastest energy layer switching, optimized spot sizes and the Adaptive Aperture proton multi-leaf collimator system. This capability provides dose gradient advantages with up to a three times sharper lateral penumbra, reducing dose uncertainty at the edge of the tumor, thus sparing healthy tissue and preventing unnecessary radiation to sensitive locations. This improved precision in delivery is combined with hyper-fast beam delivery, reducing the sensitivity to motion that current PBS technologies face when treating moving lesions such as those in the thoracic cavity.
The S250i system is based on Mevion’s high-efficiency, low-financial-risk S250 Series platform. The core technology of the S250 Series includes what the company calls the world’s only gantry mounted superconducting synchrocyclotron, a six degree-of-freedom treatment couch and advanced in-room image-guided radiation therapy (IGRT), all leveraged in the S250i system.
The Mevion S250i lowers the cost per patient by reducing capital and operating costs and increasing treatment throughput. Mevion customers have achieved the fastest per-room patient ramp-up in the history of proton therapy, according to the company. High throughput is achieved via linear accelerator (linac)-like workflow, low maintenance requirements and high uptime.
For more information: www.mevion.com


 January 30, 2026
January 30, 2026 









