August 19, 2019 — Lunit has announced Korea Ministry of Food and Drug Safety (MFDS) approval of its artificial intelligence (AI) solution for breast cancer, Lunit Insight MMG. The announcement marks the second approval of its AI solution by Korea MFDS since 2018, when Lunit had gained initial regulatory clearance of its AI software.
Lunit Insight MMG, which is now available for domestic sales in Korea, runs on Lunit’s proprietary AI technology, co-developed with Korean medical institutions including Yonsei Severance Hospital, Asan Medical Center and Samsung Medical Center.
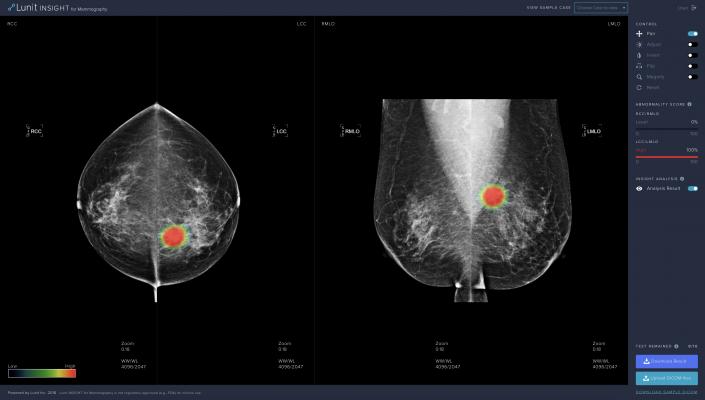
The software analyzes mammography images, with an indication of the location of lesions suspicious for breast cancer and an abnormality score of the detected lesion.
According to Lunit CEO Brandon Suh, breast cancer is one of the most common female cancer, taking up to 24 percent of all female cancer cases. He added that among the patients suspicious of breast cancer after screening mammography, only 29 percent are actually diagnosed with cancer after biopsy.
Suh said that the company is using AI to increase the effectiveness of mammography screening and reduce unnecessary biopsies. According to the company, the algorithm is designed to assist prompt and accurate diagnosis by physicians, by showing only malignant lesions and automatically ignoring benign lesions. It has been trained with more than 200,000 mammography cases, which includes 50,000 breast cancer cases.
Lunit provides an online demo for users to test the performance of its software.
The company had previously received MFDS clearance in August 2018 for its AI solution for chest X-ray. The software is currently being used at multiple hospitals and medical examination centers throughout the country, including Seoul National University Hospital.
For more information: www.lunit.io


 March 06, 2026
March 06, 2026 









