August 27, 2018 — iSchemaView announced that more than 575 stroke centers in 22 countries have selected the RAPID advanced imaging platform for cerebrovascular imaging, with 520 currently installed. RAPID technology assists physicians in the analysis of brain images using automated tools for CT ASPECTS, computed tomography (CT) angiography, CT perfusion, magnetic resonance (MR) diffusion and perfusion for more than 85,000 stroke cases per year. RAPID is also currently deployed in six multi-center clinical trials globally.
Under institutional review board (IRB) approval, iSchemaView’s RAPID platform was recently used to select patients for two landmark stroke trials published in The New England Journal of Medicine, DAWN and DEFUSE 3. Both trials successfully treated stroke patients up to twenty-four hours after onset. RAPID was the exclusive imaging tool used to aid in patient selection in both studies. The results of the studies helped change the American Heart Association and American Stroke Association’s stroke guidelines to include CT perfusion and MR perfusion.
The prior treatment window for mechanical thrombectomy was up to six hours. Starting in 2018, select patients with salvageable brain tissue identified through advanced imaging are now eligible for treatment up to twenty-four hours after they were last seen well.
iSchemaView Senior Vice President Anil Singhal, M.D., noted that several large hospital systems have recently adopted the RAPID platform, including 17 hospitals of Baylor Scott & White Health. Singhal said that by deploying RAPID through in a hub and spoke model, more patients will be able to get the right treatment at the most appropriate facility, vastly improving patient care and at the same time increasing hospital efficiency.
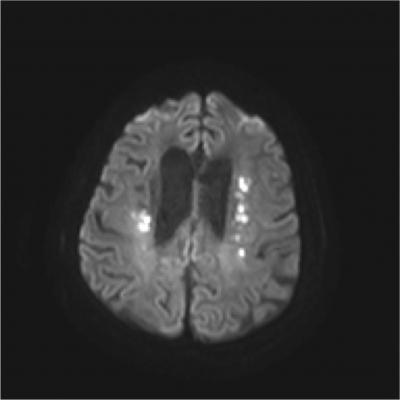
The RAPID neuroimaging platform creates high-quality images from non-contrast CT, CT angiography, CT perfusion, and MRI diffusion and perfusion studies. The software provides an intuitive and easily interpretable real-time view of brain perfusion, allowing physicians to determine lesion volumes for a wide variety of different thresholds.
The platform includes four different imaging products, tailored to the particular needs of each facility:
- RAPID MRI provides fully automated, easy-to-interpret diffusion and perfusion maps that identify brain areas with low ADC values, as well as delayed contrast arrival. RAPID MRI perfusion automatically quantifies regions of reduced cerebral blood flow, volume and transit time that exceed pre-specified thresholds;
- RAPID CTP provides CT perfusion maps that automatically quantify regions of reduced cerebral blood flow, volume and transit time that exceed pre-specified thresholds. Regions are color coded, and the volumes of interest are automatically measured. Maps (including mismatch maps) of the severity of Tmax delays are provided using a four-color-coded scale;
- RAPID CTA automatically provides clear, easy-to-interpret CTA maps which include a colored overlay to identify brain regions with reduced blood vessel density. The severity of reduction can be readily visualized by a simple four-color-coded scale. Additionally, a 3-D reconstruction of the vasculature allows physicians to rotate the image for optimal viewing of the vessels from multiple angles; and
- RAPID ASPECTS automatically generates a standardized score — based on clinically validated machine learning algorithms — that enables physicians to easily communicate about the extent of a patient’s ischemic changes and to determine eligibility for thrombectomy (clot removal). In addition, RAPID ASPECTS provides clear visualization of the brain so that clinicians can better scrutinize each region and confirm the automated score. RAPID ASPECTS is CE Marked.
For more information: www.i-rapid.com


 February 06, 2026
February 06, 2026 









