August 8, 2018 — iCAD announced U.S. Food and Drug Administration (FDA) clearance of its latest artificial intelligence (AI) software solution, PowerLook Density Assessment Version 3.4. The software is compatible with iCAD’s digital breast tomosynthesis (DBT) platform on both GE and Hologic mammography systems, enhancing the company’s comprehensive suite of breast health solutions available in a single platform.
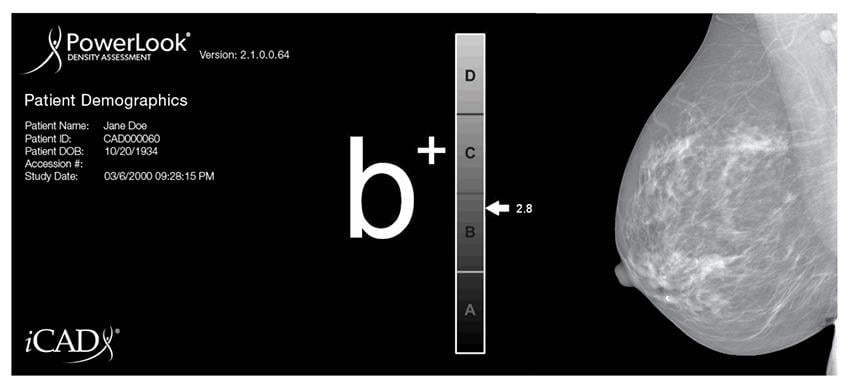
Breast density plays a critical role in both risk assessment and accuracy, as mammography sensitivity is reduced to approximately 48 percent from an average of 98 percent in those women with dense breasts, according to iCAD CEO Ken Ferry. iCAD’s technology rapidly produces consistent breast density results while reducing the risk of reader variability, enabling radiologists to more easily identify women who might benefit from additional screening.
According to a recent study published in the Annals of Internal Medicine, between 40-50 percent of women in the United States are considered to have dense breasts, which can increase tumor aggressiveness, as well as mask the presence of tumors in a mammogram.
iCAD’s PowerLook Density Assessment 3.4 delivers automated, rapid and reproducible assessments of breast density to help identify patients that may experience reduced sensitivity to digital mammography due to dense breast tissue. This technique, based on machine learning, calibrates the patient’s breast density to the appropriate category corresponding to the American College of Radiology's Breast Imaging Reporting and Data System (BI-RADS) reporting system.
“Since implementing PowerLook Density Assessment, our radiologists have been able to provide more consistent breast density results for our patients during screenings. We must consider many aspects of each patient’s case at once, but PowerLook Density Assessment allows us to focus on our primary goal of finding breast cancer. Density matters and we believe the greater accuracy of assessment with iCAD’s PowerLook Density Assessment will result in greater patient satisfaction and improve patient care,” stated Beth Ingram, M.D., radiology, interventional at Reid Health, Indiana.
For more information: www.icadmed.com
Reference


 February 13, 2026
February 13, 2026 









