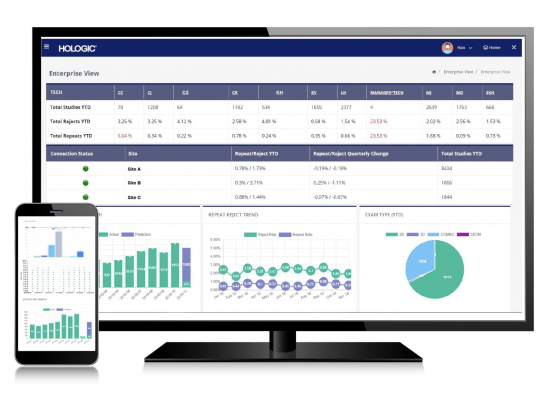
November 10, 2020 — Hologic, Inc. announced innovative updates to its Unifi Analytics platform, a breakthrough business intelligence tool that provides mammography centers insights into technologist performance, proactive device maintenance, and business considerations.
Unifi Analytics 1.2 enables users to better understand and improve daily mammography workflow by providing valuable insights regarding volume and time spent on screening, diagnostic and biopsy studies. Additionally, facilities can now benchmark efficiency metrics against a comprehensive database of more than 2,500 Hologic gantries across the nation to identify opportunities for improvement. The platform also includes new features focused on paddle utilization and compression force. These features can help users improve staff performance in ways that lead to better image quality and patient satisfaction.
“As the global leader in breast health, ongoing product innovation is central to our commitment to provide cutting-edge, insight-driven solutions that improve outcomes for patients and reduce costs and improve efficiencies for healthcare facilities,” said Jennifer Meade, Hologic’s Division President, Breast and Skeletal Health Solutions. “These enhancements to Unifi Analytics, which were informed by research and customer feedback, will provide imaging centers with even more actionable insights so they have a clear path to improve overall performance.”
Initially launched in 2019, Unifi Analytics equips administrators with the modern-day tools they need to make informed business decisions, allowing imaging centers to maximize efficiency and reduce downtime in the mammography suite. By tracking the installed base of mammography devices at a facility, the web-based platform delivers statistical analyses of factors such as technologist efficiency and quality. It also enables imaging centers to maximize device utilization, identify potential risks and challenges, and benchmark performance against the national average of Hologic users. Additionally, Unifi Analytics employs advanced machine intelligence to predict end-of-life tube failures before they occur, allowing facilities to avoid costly unplanned downtime.
For more information: www.hologic.com


 March 06, 2026
March 06, 2026 









