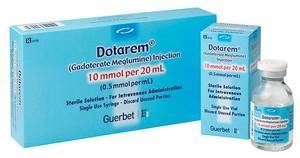
April 21, 2020 — Guerbet announced that it received U.S. Food and Drug Administration (FDA) approval to manufacture Dotarem (gadoterate meglumine) injection at its Raleigh, N.C., facility. Until late 2019, Dotarem was manufactured exclusively outside of the United States.
Guerbet intends to produce Dotarem within the United States for US-based customers, in alignment with Guerbet’s broader vision to supply US-manufactured products to US customers. Guerbet’s line of power injectors and urology systems are manufactured and/or assembled in Cincinnati, Ohio.
“We are proud to add Dotarem to our list of products manufactured or assembled in the United States for US customers,” reported Thomas McLaughlin, vice president, North America. The Raleigh facility is manufacturing Dotarem in both vials and plastic syringes. The plastic syringes feature a new pushrod that is designed to make hand injections more ergonomic, and plastic pre-filled syringes reduce patient risks including the chance of contamination as compared to bulk fill containers. The StarClip adapter enables power injection of Dotarem (in the new plastic syringes) using Guerbet’s OptiStar Elite injector. The new, more durable outer packaging allows for both improved convenience and workflow efficiency in the radiology suite.
As a result of U.S. manufacturing approval, customers should refer to the following new product item numbers and NDC codes when ordering new product inventory:
For Dotarem® 10x15mL Vials:
New Item Code: 368220012
New NDC Code: 67684200102
For Dotarem 10x100mL Vials:
New Item Code: 368220014
New NDC Code: 67684200104
For Dotarem 10x20mL Vials:
New Item Code: 368220013
New NDC Code: 67684200103
For Dotarem 20mL Plastic Prefilled Syringe:
New Item Code: 368230013
New NDC Code: 67684300103
All units of measure remain the same except for the Prefilled Syringe (PFS) product line, which has changed from 5/box to 10/case.
These are the first of several SKUs to launch in the U.S. Remaining SKUs will be phased in throughout 2020.
Customers are encouraged to continue using their existing supply of glass syringes with Dotaclip as new syringes with StarClip are introduced. Customers should work with their distribution partners to ensure that correct products are received throughout this transition process.
For more information: www.guerbet.com


 March 03, 2026
March 03, 2026