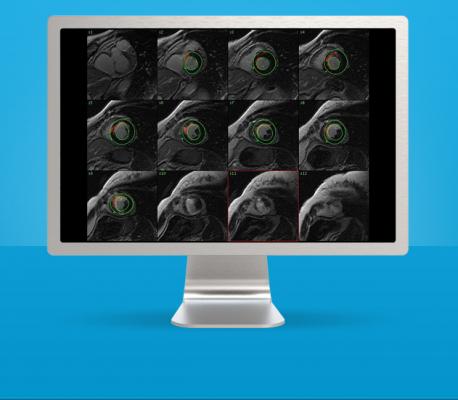
GE Healthcare's CardiacVX
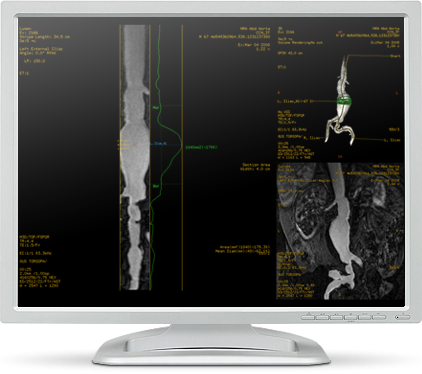
GE Healthcare's MR VesselIQ Xpress

GE Healthcare's CardiacVX

GE Healthcare's MR VesselIQ Xpress
February 6, 2013 — GE Healthcare announced two new software packages for advanced analysis of cardiovascular magnetic resonance (MR) images — CardiacVX and MR VesselIQ Xpress. Both CardiacVX and MR VesselIQ Xpress were featured in the GE Healthcare booth at the annual meeting of the Society for Cardiac Magnetic Resonance (SCMR) on Feb. 1-2, 2013, in San Francisco, Calif.
Cardiac MR can provide a wealth of information about the patient’s cardiac condition such as functional parameters, flow, and infarction. However, extracting this information and presenting it in an integrated fashion to the referring physician can be a time-consuming task for the technologist or clinician performing the study. GE Healthcare’s new CardiacVX software delivers fast, intuitive, semi-automatic analysis of key cardiac parameters, allowing the user to create a comprehensive cardiac patient report within a streamlined clinical workflow.
Recently the U.S. Food and Drug Administration (FDA) 510(k) cleared, CardiacVX runs on GE Healthcare’s Advantage Workstation (AW), and provides a range of reproducible tools for rapid reviewing and reporting. For example, CardiacVX performs fast left ventricle segmentation and volumetric functional analysis in just two clicks. Additional tools include myocardial infarct evaluation, flow analysis (with Qp/Qs ratio), time-course analysis, iron-overload assessment, patent foramen ovale (PFO) assessment and one-click customizable reporting macros, all within an intuitive, guided user interface.
“CardiacVX was designed to address the clinical demand for a more automated and user-friendly cardiac post-processing tool to allow clinicians to quickly and easily arrive at needed diagnostic information,” said Anja Brau, Ph.D., manager of global cardiac MR applications for GE Healthcare.
Computed Tomography (CT) Angiography has been widely used for assessing vascular stenosis or abnormalities, but increasingly, MR Angiography is utilized due to the lack of radiation and calcium blooming artifacts. Being able to use similar analysis workflow between CT and MR can greatly improve the clinician’s diagnostic efficiency. MR VesselIQ Xpress, built based on GE Healthcare’s CT VesselIQ Xpress platform, is an optimized image analysis package for MR angiographic data to efficiently analyze selected vessels for stenosis, directional tortuosity and other vascular anomalies. MR VesselIQ Xpress provides advanced tools such as automatic vessel tracking with centerline display of any vessel, quick 3-D visualization and fast access to vessel cross section and profile images.
In addition, MR VesselIQ Xpress automatically provides size, stenosis and length measurements of abnormal anatomical structures with two deposited points. Measurement tables and associated images are captured and ready to be included in reports. This software was designed to assist radiologists and other clinicians in the efficient evaluation and assessment of vascular anatomy and disease.
CardiacVX and MR VesselIQ Xpress software packages run on GE’s AW and are compatible with AW releases 4.4, 4.5, and 4.6.
GE Healthcare is committed to humanizing MR by focusing on the needs of the patient, technologist and clinician. These are the latest offerings in a series of products designed to improve cardiovascular exam processing for the technologist and clinician while maximizing patient care — in the true spirit of Humanizing MR.
For more information: www.gehealthcare.com




 February 13, 2026
February 13, 2026 









