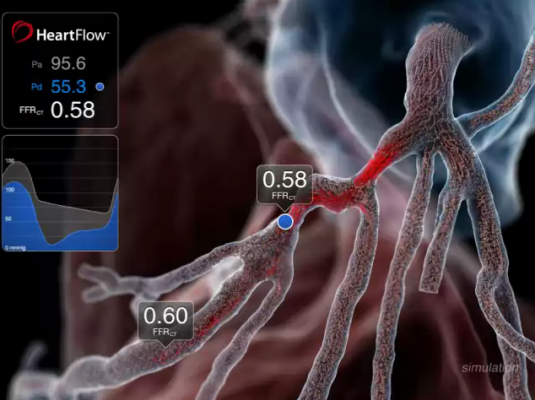
August 9, 2017 — HeartFlow Inc. announced that four Blue Cross Blue Shield companies have each issued a positive medical policy for the HeartFlow FFRct (fractional flow reserve-computed tomography) Analysis.
Each of the four health plans — Horizon Blue Cross Blue Shield of New Jersey (Horizon BCBCNJ), Blue Cross Blue Shield of Arizona, Blue Cross of Idaho and Blue Cross Blue Shield of Kansas City — assessed the technical performance, diagnostic accuracy, clinical utility and clinical benefits of the HeartFlow FFRct Analysis for patients with suspected coronary artery disease (CAD). They determined that the use of noninvasive FFRct following a positive coronary computed tomography (CT) angiogram is considered medically necessary to guide decisions about the use of invasive coronary angiography for its members with stable chest pain (i.e., those with suspected or presumed stable ischemic heart disease).
CAD, which today affects 16.8 million Americans, develops when the coronary arteries narrow, reducing blood flow to the heart and causing angina, myocardial infarction and death. The HeartFlow FFRct Analysis is the first and only non-invasive technology to provide insight into both the extent of CAD and the impact of the disease on blood flow to the heart. It is designed to help enable clinicians design a definitive, personalized treatment plan for each patient and reduce the need for additional invasive or noninvasive testing.
HeartFlow CEO John H. Stevens, M.D., said the decision by Blue Cross Blue Shield, along with the American Medical Association’s decision to issue a set of Category III CPT codes for HeartFlow FFRct Analysis, indicates increasing acceptance and adoption of the technology.
Several other payers have also issued positive coverage decisions, including Aetna, which covers more than 23 million lives. In June, Evidence Street, which provides healthcare technology evaluations for the Blue Cross Blue Shield Association, a national federation of 36 independent Blue Cross and Blue Shield companies that collectively provide healthcare coverage for 105 million Americans, issued a review supporting the HeartFlow FFRct Analysis and indicating an expected improvement in net health outcomes. Earlier this year, the National Institute for Health and Care Excellence (NICE) of the U.K.’s National Health Service, which covers 59 million lives, issued positive guidance, recommending the HeartFlow FFRct Analysis to help determine the cause of stable chest pain.
Additionally, the American College of Cardiology (ACC) and American Heart Association (AHA) recently released updated Appropriate Use Criteria for Coronary Revascularization in Patients with Stable Ischemic Heart Disease, which include the use of HeartFlow FFRct Analysis in determining the appropriateness of revascularization in many clinical scenarios.
For more information: www.heartflow.com


 February 13, 2026
February 13, 2026 









