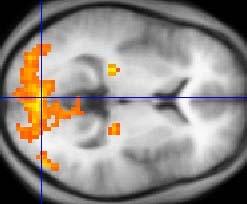
May 19, 2011— GE Healthcare announced U.S. Food and Drug Administration clearance of Ready View, a new magnetic resonance imaging (MRI) advanced visualization platform to help clinicians process and analyze images anytime and anywhere. A part of the Dexus workflow, Ready is accessible through any PC, PACS or RIS workstation, allowing access to process and analyze images in any office, meeting room or even at home.
The Ready View advanced visualization platform provides a combination of protocols, applications and advanced tools that enable a fast, easy and quantified analysis. In addition to standard and advanced protocols, such as 4-D review and image averaging, Ready View offers fast and accurate multiparametric protocols, such as brain oncology, knee, liver and many more. Multiparametric protocols offer a new, simple and intuitive workflow to process all functional data from a single screen without having to leave a reading station.
Combining Ready View’s robust accessibility with its many productivity tools such as auto-processing, save state, one-click motion correction, and real time thresholding and segmentation, Ready View will not only help streamline image processing and analysis but will also help clinicians provide an effective diagnosis.
For more information: www.gehealthcare.com


 February 13, 2026
February 13, 2026 









