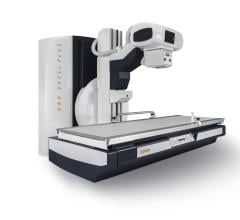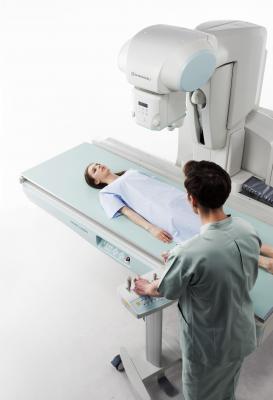
FDA Clears Shimadzu Sonialvision G4 Universal R/F Imaging system
April 30, 2014 — Shimadzu Medical Systems USA has announced that the Sonialvision G4 universal digital R/F table system has received 510(k) clearance from the U.S. Food and Drug Administration (FDA). The Sonialvision G4 expands Shimadzu’s product portfolio with the addition of the highest performance most sophisticated system that has received strong acceptance already in Europe and Asia.
The Sonialvision G4 is designed to perform a wide variety of examinations — angiography, endoscopy, video fluoroscopy for gastrointestinal series and swallowing exams, orthopedic exams and general radiography. It acquires high image quality images rapidly and uses low radiation dose levels. Its new ergonomic design is patient safety- and hygiene-focused, accommodates all sizes of ambulatory and stretcher/wheelchair-confined patients, and offers workflow-efficiency features to help improve technologist productivity.
The system combines a 17- x 17-inch flat panel detector (FPD) with Shimadzu’s next generation digital imaging platform. Its 139 ?m ultra-small pixel pitch and high contrast maximizes the FPD performance. Its easily switchable large-to-small fields-of-view cover a range of imaging from finger tips to the entire chest or abdomen. Also, the wide 202.5-centimeter movement range of the Sonialvision G4 can capture images of the entire body without moving the patient.
Various real-time digital filtering processes effectively suppress halation near skin surfaces and around shadows where organs overlap. Smoother imaging is achieved with no delay or image lags by applying optimal digital filtering and extracting noise components. Used in long spine and limb imaging, Shimadzu’s Slot Beam functionality is available as an advanced application option. With its lower dose profile, Slot Beam is an excellent choice for pediatrics. The Sonialvision G4 is equipped with a wealth of functions to facilitate urological exams. The edge of the imaging range can be positioned as close as 9.5 centimeters from the head end of the table, given a 17-inch field-of-view, making it easy to perform procedures using an optical endoscope. It is also possible to tilt the table without changing an observation position. A specialized collimation function helps reduce dose.
An oblique projection function prevents overlapping organs when performing gastrointestinal examinations. Fluoroscopic images can be saved in digital imaging and communications in medicine (DICOM) format. Patients in wheelchairs can be examined simply by extending the imaging chain, which has a maximum 15 centimeter per second quick movement.
The Sonialvision G4 supports angiography procedures with its wide clean design, and its large field of view enables digital subtraction angiography (DSA) to be used for examinations ranging from the hepatic artery to the entire lower extremities.
Special features for every type of exam the Sonialvision G4 performs help to minimize radiation dose exposure levels to the patient. A table that can support a patient weighing up to 700 lb (318 kg) in the horizontal position for bariatric procedures is seamless for easy cleaning and incorporates safety features to facilitate safe access and egress by patients.
This new generation system design of the G4 also provides productive and comfortable workflow to enhance convenience and operability for the technologists who use it. Its high-speed dual processor can simultaneously resolve images, register patient information, and perform other functions quickly without interfering with the examination being performed. The number of operating steps a technologist needs to perform during an examination is significantly reduced because the X-ray generator, R/F table and digital processor are linked. Shimadzu dealers throughout the United States and in the Caribbean are now accepting orders for the Sonialvision G4.
For more information: www.shimadzu.com


 August 14, 2025
August 14, 2025