May 18, 2023 — Cassling announced it is a new strategic sales agent in various geographic territories across the United States for Radiaction Medical, the makers of a novel, FDA-cleared advanced radiation blocking technology.
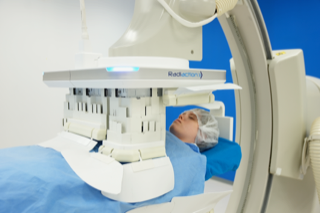
The Radiaction system is the first and only advanced technology to proactively block radiation at the source, providing comprehensive, head-to-toe radiation protection to the entire interventional team. Radiaction provides an unparalleled level of protection to physicians and staff while allowing unencumbered patient care, access and service.
“Radiaction is a natural fit for a partnership with Cassling,” said Elizabeth Grieger, VP of Growth and Innovation at Cassling. “For nearly 40 years, our hospital partners, including interventional suites and catheterization labs that enhance patient outcomes, have trusted us to connect them to state-of-the-art systems.”
Grieger continued, “When we met with Radiaction, we recognized how its technology is truly a revolution in what we can do to protect providers in these settings. I’m excited that we can provide our customers with an advanced solution that is designed to protect the treating physicians, as well as everyone in the procedure room. Radiaction seamlessly integrates into procedural workflow, giving providers the protection they deserve while still enabling them to do their best work for patients.”
Traditionally, procedures completed in interventional suites and catheterization labs have required physicians and other team members in the vicinity of the patient to don heavy lead aprons to protect themselves from radiation scatter. While a good start, lead gowns still provide gaps in radiation protection and leave key areas of the body, such as the head, face, legs and arms exposed.
Radiaction’s device, however, provides full-body protection. As an accessory to existing C-arm imaging equipment, Radiaction blocks more than 90% of scatter radiation to the entire room, with up to 97% radiation scatter protection for the physician’s head, according to clinical studies. The device fully encapsulates the imaging X-ray beam, and at the touch of a button, the system dynamically deploys or retracts within seconds, accommodating any patient size, imaging angulation or location while enabling the team to maintain immediate access to their patients throughout the procedure.
Jonathan Yifat, Radiaction Medical’s CEO, is excited about the partnership’s potential: “When we seek industry partners for a collaborative effort, capabilities are important, but shared corporate values are the best indicator of a productive relationship,” he said, adding, “Cassling’s deep commitment to improving the workplace experience and safety of frontline healthcare providers makes them an obvious choice for us. We are especially eager to work with them to introduce the groundbreaking Radiaction technology to Cassling’s customers, to protect and serve the people who care for patients.”
Radiaction has received 510(k) clearance from the Food and Drug Administration (FDA) to enable compatibility with certain C-arm machines. As part of an initial agreement, Cassling will offer the system to cardiology lab customers throughout the country.
For more information: www.cassling.com
Related Radiation Scatter Content:
Radiation Scatter 101: Risks, Dangers and Latest Solutions
Market for MRI-based Radiotherapy Could Soon Widen
Carestream Introduces SmartGrid Software to Reduce Effects of Scatter Radiation


 February 04, 2026
February 04, 2026 









