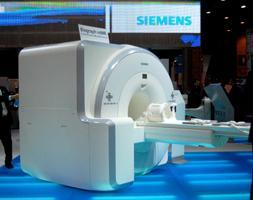
June 10, 2011 – The U.S. Food and Drug Administration (FDA) granted Siemens Healthcare 510(k) clearance for the Biograph mMR, the first system worldwide to enable simultaneous whole-body acquisition of data from magnetic resonance (MR) and positron emission tomography (PET). While MR provides exquisite morphological and functional details in soft tissue, PET goes further to investigate the human body at the level of cellular activity and metabolism. The Biograph mMR enables users to generate the location, function and metabolic activity of organs in a single image at the same time. The Biograph mMR allows a combined approach to imaging anatomical, functional and biochemical characteristics of disease. Potential clinical applications for molecular MR include the early identification and staging of malignancies, therapy planning and treatment. The Biograph mMR shows the greatest promise in oncologic and neurologic applications where MR and PET have proven their clinical value. The Biograph mMR has been adopted as a diagnostic imaging research tool for developing novel approaches to the understanding, diagnosis, and treatment of disease. Initial Biograph mMR installations are at the University Hospital in Tuebingen and the University Hospital “Klinikum rechts der Isar” of the Munich Technical University, both in Germany; and Boston’s Massachusetts General Hospital. "We’ve initiated clinical use testing of Biograph mMR in an effort to diagnose diseases at a very early stage; to see the progression of disease and to use that information to develop a therapy plan precisely focused on the respective patient. Furthermore, we plan to use the system for cancer followup in the long run, by reducing radiation exposure by the use of the system,” said Prof. Dr. Markus Schwaiger, director of the Clinic for Nuclear Medicine at the “Klinikum Rechts der Isar” of the Munich Technical University. Prior to the Biograph mMR, the integration of MR and PET technologies was nearly impossible. The conventional PET detectors, which use photomultiplier tubes, could not be used in the strong magnetic field generated by an MR system. Integration was further limited by the lack of space inside the MR device. For this reason, previous MR-PET fusion imaging solutions relied not on simultaneous imaging, but rather sequential imaging (or post-scan registration) of MR and PET data, resulting in a significant time lag. The Biograph mMR represents the first complete integration of diagnostic-grade MR and PET into a single-gantry whole-body scanner through incorporation of its 3.0T MR technology and unique Avalanche photodiode solid-state PET detector technology, which addresses the functional incompatibility of MR and PET. With the Biograph mMR, patients can be scanned in as few as 30 minutes for a combined whole-body exam, compared to one hour or more for sequential MR and PET exams. Additionally, Biograph mMR has a footprint comparable to a standard, high-field MR scanner and can be sited in a typical MR room, eliminating renovation costs for facilities seeking to replace an existing MRI. Earlier this year, Siemens Healthcare received the 2011 North American Frost & Sullivan Award for New Product Innovation for the Biograph mMR. The Growth Partnership Company presented the award to Siemens for demonstrating excellence in the following categories: innovative element of the product, leverage of leading-edge technologies, value-added features/benefits, increased customer return on investment (ROI) and customer acquisition/penetration potential. For more information: www.siemens.com/healthcare


 February 13, 2026
February 13, 2026 









