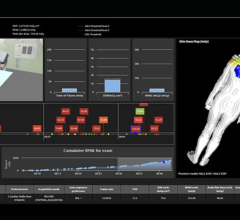
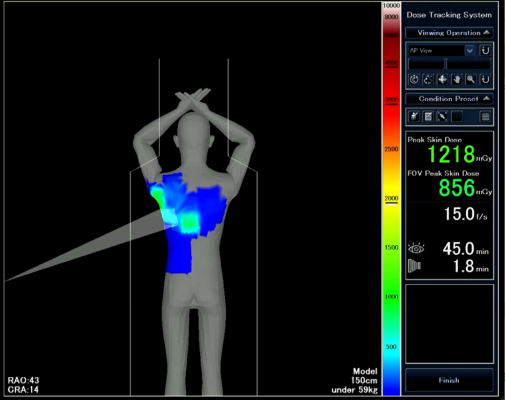
January 20, 2014 — Toshiba America Medical Systems’ Dose Tracking System allows clinicians to track X-ray skin dose exposure in real time during interventional procedures. The U.S. Food and Drug Administration cleared Toshiba’s Infinix-I cardiovascular X-ray systems for use in pediatric and adult cardiac and abdominal procedures.
Toshiba’s Dose Tracking System displays live and cumulative
radiation exposure through an color-coded indicator on a 3-D visualization of the patient. The display shows where radiation is being administered on the patient’s body in real time. The technology is intended to help clinicians appropriately distribute skin doses and minimize risk of locally concentrated high exposure.
The Dose Tracking System was developed in a partnership with the University of Buffalo.


 February 04, 2026
February 04, 2026