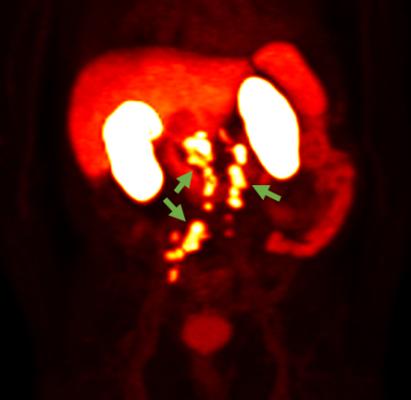
September 28, 2022 — Blue Earth Diagnostics, a Bracco company and recognized leader in the development and commercialization of innovative PET radiopharmaceuticals, today announced that the U.S. Food and Drug Administration (FDA) has accepted its filing for a New Drug Application (NDA) for 18F-rhPSMA-7.3, an investigational radiohybrid Prostate-Specific Membrane Antigen-targeted (PSMA) PET imaging agent. The submission is for the use of 18F-rhPSMA-7.3 PET for diagnostic imaging of prostate cancer.
“This event marks a significant milestone in advancing our robust prostate cancer portfolio, and we are very pleased that the FDA has accepted our NDA submission for the use of 18F-rhPSMA-7.3 PET imaging in prostate cancer patients,” said David E. Gauden, D.Phil., Chief Executive Officer of Blue Earth Diagnostics. “We look forward to working with the Agency throughout the review process, with the goal of having an approved product that is widely available and accessible across the United States. Subject to FDA approval, we believe that 18F-rhPSMA-7.3 PET imaging may be clinically useful in the management of men affected by prostate cancer across the care continuum. All of us at Blue Earth want to express our sincere gratitude to the many patients, physicians, clinical trial sites and collaborators who have worked closely with us to progress 18F-rhPSMA-7.3, and to having successfully completed our Phase 3 clinical trials despite all the challenges presented by the COVID-19 pandemic.”
The submission is supported by clinical data from one prospective Phase 1 study and two prospective Phase 3 clinical trials sponsored by the Company. Blue Earth Diagnostics’ Phase 3 LIGHTHOUSE trial assessed the safety and diagnostic performance of 18F-rhPSMA-7.3 PET in men with newly diagnosed prostate cancer in 356 patients. Key results from the LIGHTHOUSE trial will be presented at an upcoming medical conference. The Phase 3 SPOTLIGHT trial evaluated the use of 18F-rhPSMA-7.3 in 391 men with suspected prostate cancer recurrence based on elevated PSA level. Results from the SPOTLIGHT study have been presented at medical meetings earlier this year. Further clinical support for the submission included results published by the Technical University of Munich.
“Prostate cancer is a leading cause of male cancer-related death worldwide, and accurate localization and staging of the disease is critical in establishing optimal medical management strategies,” said Eugene Teoh, MBBS, MRCP, FRCR, D.Phil., Chief Medical Officer of Blue Earth Diagnostics. “We believe that the performance of 18F-rhPSMA-7.3, its high PSMA binding affinity and potential for low bladder activity will make it a valuable diagnostic tool that is radiolabeled with 18F for high image quality and readily available patient access.”
About Prostate Cancer
Prostate cancer is the second most frequently diagnosed cancer in men and the fifth leading cause of death worldwide. It is most commonly diagnosed in men aged 65 years and over. In its early stages, it is largely asymptomatic and tumors are detected by increased prostate-specific antigen (PSA) levels in the blood. Effective staging of newly diagnosed prostate cancer − determining its extent and whether it may have metastasized − is critical in assessing a patient’s prognosis and informing individual clinical management strategies. Up to 25% of prostate cancer patients may have detectable lymph node metastases, which are correlated with a risk for recurrence and decreased overall survival. Regardless of initial therapy, there is potential for recurrence of prostate cancer. Up to one-third of men treated for primary prostate cancer will experience a biochemical recurrence (BCR) within 10 years, and one-third of men experiencing recurrence will develop metastatic disease within 8 years. Up to 40% of patients who undergo radical prostatectomy, and up to 50% of patients who undergo radiation therapy will develop local or distant recurrences within 10 years. Recurrent prostate cancer is clinically challenging, given that its natural history is highly variable.
About 18F-rhPSMA-7.3 and Radiohybrid Prostate-Specific Membrane Antigen (rhPSMA)
18F-rhPSMA-7.3 is an investigational agent that consists of a radiohybrid Prostate-Specific Membrane Antigen (PSMA)-targeted receptor ligand which attaches to and is internalized by prostate cancer cells, and is labeled with the 18F radioisotope for PET imaging. rhPSMA compounds are referred to as radiohybrid (“rh”), as each molecule possesses three distinct domains. The first consists of a Prostate-Specific Membrane Antigen-targeted receptor ligand which attaches to and is internalized by prostate cancer cells. It is attached to two labelling moieties which may be radiolabeled with either 18F for PET imaging, or with isotopes such as 177Lu or 225Ac for therapeutic use – creating a true theranostic technology. Radiohybrid technology and rhPSMA originated from the Technical University of Munich, Germany. Blue Earth Diagnostics acquired exclusive, worldwide rights to rhPSMA diagnostic imaging technology from Scintomics GmbH in 2018, and therapeutic rights in 2020, and has sublicensed the therapeutic application to its sister company Blue Earth Therapeutics. Currently, rhPSMA compounds are investigational and have not received regulatory approval.
About the Phase 3 LIGHTHOUSE Clinical Trial for 18F-rhPSMA-7.3
The Phase 3 LIGHTHOUSE clinical trial was a prospective, Phase 3, multi-center, single-arm, imaging study investigating the safety and diagnostic performance of 18F-rhPSMA-7.3 Positron Emission Tomography (PET) in men with newly diagnosed prostate cancer. The study evaluated 356 patients at clinical sites in the United States and Europe. Additional information about the Phase 3 LIGHTHOUSE trial is available at www.clinicaltrials.gov (NC04186819).
About the Phase 3 SPOTLIGHT Clinical Trial for 18F-rhPSMA-7.3
The SPOTLIGHT Phase 3 clinical trial was a prospective, Phase 3, multi-center, single-arm, imaging study investigating the safety and diagnostic performance of 18F-rhPSMA-7.3 Positron Emission Tomography (PET) in men with suspected prostate cancer recurrence based on elevated Prostate-Specific Antigen (PSA) following prior therapy. The study evaluated 391 patients at clinical sites in the United States and Europe. Additional information about the Phase 3 SPOTLIGHT trial is available at www.clinicaltrials.gov (NCT04186845).
For more information: www.blueearthdiagnostics.com
Related Content:
Blue Earth Diagnostics Announces Key Results from Phase 3 SPOTLIGHT Study of 18F-rhPSMA-7.3


 February 04, 2026
February 04, 2026 









