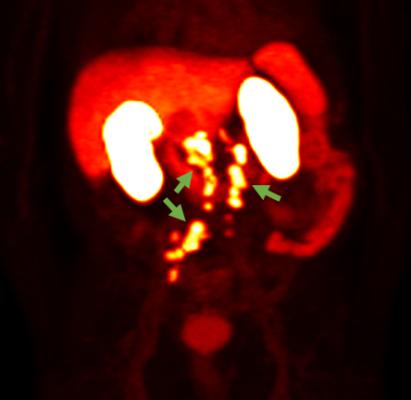
18F-rhPSMA-7.3 PET Photo courtesy of Blue Earth Diagnostics. General use: This 18F-rhPSMA-7.3 PET image shows prostate cancer spread beyond the prostate region. Technical use: This 18F-rhPSMA-7.3 PET image shows detection of recurrent prostate cancer in several retroperitoneal lymph nodes, as indicated by the green arrows.
February 18, 2022 — Blue Earth Diagnostics, a Bracco company and recognized leader in the development and commercialization of innovative PET radiopharmaceuticals, announced publication of key results from its Phase 3 SPOTLIGHT trial of 18F-rhPSMA-7.3 in recurrent prostate cancer in an abstract being presented at the ASCO 2022 Genitourinary Cancers Symposium (ASCO GU). 18F-rhPSMA-7.3 is an investigational Prostate-Specific Membrane Antigen-targeted radiohybrid (rh) PET imaging agent. The SPOTLIGHT study (NCT04186845) is a Phase 3, multi-center, single-arm imaging study, conducted in the United States and Europe to evaluate the safety and diagnostic performance of 18F-rhPSMA-7.3 PET imaging in men with suspected prostate cancer recurrence based on elevated PSA following prior therapy.
Based on the majority read results from the three blinded, independent PET readers, the overall detection rate (DR) of 18F-rhPSMA-7.3 PET in the SPOTLIGHT study was 83% (322/389). When stratified by PSA level, the DRs in the 305 patients with prior prostatectomy were: PSA <0.5 ng/mL: 64% (77/120); PSA ≥0.5 and <1 ng/mL: 76% (51/67); PSA ≥1 and <2 ng/mL: 93% (41/44); PSA ≥ 2 and <5 ng/mL: 96% (43/45); PSA ≥ 5 and <10 ng/mL: 88% (14/16); and PSA ≥10 ng/mL: 100% (13/13).
The study was designed to confirm positive 18F-rhPSMA-7.3 PET imaging findings using a composite Standard of Truth (SoT) consisting of either: histopathology (considered the gold standard); or conventional imaging (primarily with CT, bone scan or MRI, with fewer procedures using 18F-fluciclovine PET). In 366 men with a composite SoT (histopathology and/or conventional imaging), the patient-level Correct Detection Rate (CDR) was 57% (95% CI, 52-62). The region-level Positive Predictive Value (PPV) was 60% (55-65). However, in the subset of patients using the preferred gold standard of histopathology as the SoT (n=69), the patient-level CDR and region-level PPVs were much higher, at 81% (70-90) and 72% (63-81), respectively, providing important evidence regarding 18F-rhPSMA-7.3’s performance. Additional endpoints, including patient-level PPV and the percentage of patients upstaged by 18F-rhPSMA-7.3 PET, will be reported at future scientific meetings.
No serious adverse reactions were attributed to 18F-rhPSMA-7.3 PET in the SPOTLIGHT study. Overall, 16 (4.1%) patients had at least one treatment-emergent adverse event that was considered possibly related/related to 18F-rhPSMA-7.3. The most frequently reported events were: hypertension: 1.8% (n=7); diarrhea: 1.0% (n=4); injection site reaction: 0.5% (n=2), and headache: 0.5% (n=2).
The study will be discussed in an oral presentation at ASCO GU, “Detection rate of 18F-rhPSMA-7.3 PET in patients with suspected prostate cancer recurrence: Results from a phase 3, prospective, multicenter study (SPOTLIGHT)”, by Dr. David M. Schuster, MD, FACR, Winship Cancer Institute of Emory University, in person and online at the conference on Thursday, February 17, 2022, at 7:00 PM ET. The ASCO GU program guide is available here.
“Up to 40% of patients who undergo radical prostatectomy, and up to 50% of patients who undergo radiation therapy will develop local or distant recurrences within 10 years, and the ability to determine the extent and location of recurrent prostate cancer to inform appropriate clinical management for these men is key for physicians and their patients,” said David M. Schuster, MD, FACR, Emory University School of Medicine, and Coordinating Investigator for the SPOTLIGHT study. “Conventional imaging techniques have many limitations in prostate cancer identification and localization, and greater imaging accuracy is needed throughout the care continuum, to optimize therapeutic decision-making. The Phase 3 SPOTLIGHT study investigated the diagnostic performance of 18F-rhPSMA-7.3 PET imaging as a potential decision-making aid in assessing suspected biochemical recurrence of the disease. Taken together, we believe these strong results support the clinical utility of 18F-rhPSMA-7.3 PET in men with recurrent prostate cancer across a wide PSA range.”
“We are pleased to share these key Phase 3 SPOTLIGHT study results with the clinical community at the prestigious ASCO GU 2022 conference,” said David Gauden, D.Phil., President R&D and Chief Scientific Officer of Blue Earth Diagnostics. “SPOTLIGHT is the first of Blue Earth’s diagnostic imaging rhPSMA trials to report results, based on novel radiohybrid PSMA technology which offers potential theranostic utility in both diagnostic PET imaging and therapy. We look forward to sharing these convincing results with the U.S. Food and Drug Administration (FDA) as part of a New Drug Application for 18F-rhPSMA-7.3 PET imaging.”
Gauden continued, “Blue Earth is committed to helping men with prostate cancer across the care continuum, and we particularly wish to thank the patients and clinical teams who participated in the SPOTLIGHT study, despite the many challenges COVID-19 presented. In working to fulfill our commitment to patients, we are working on a uniquely comprehensive prostate cancer portfolio, which includes 18F-fluciclovine as well as investigational rhPSMA compounds for potential use in diagnostic PET imaging and targeted radiopharmaceutical therapy. Early studies of 18F-rhPSMA-7.3 demonstrated high binding affinity for PSMA, together with biodistribution data suggesting the potential for low bladder activity. Our focus on 18F as the isotope of choice for PET imaging with rhPSMA was made in consideration of its spatial resolution and resulting high quality of PET images, and its physical half-life which greatly facilitates ease of large-scale manufacturing and distribution for broad-based patient access.”
About Radiohybrid Prostate-Specific Membrane Antigen (rhPSMA)
rhPSMA compounds consist of a radiohybrid (“rh”) Prostate-Specific Membrane Antigen-targeted receptor ligand which attaches to and is internalized by prostate cancer cells and they may be radiolabeled with 18F for PET imaging, or with isotopes such as 177Lu or 225Ac for therapeutic use – creating a true theranostic technology. The radiohybrid technology and rhPSMA originated from the Technical University of Munich, Germany. Blue Earth Diagnostics acquired exclusive, worldwide rights to rhPSMA imaging technology from Scintomics GmbH in 2018, followed by acquisition of exclusive rights to therapeutic applications in 2020. Blue Earth Diagnostics has two Phase 3 clinical studies evaluating the safety and diagnostic performance of 18F-rhPSMA-7.3 PET imaging in prostate cancer: (“SPOTLIGHT,” NCT04186845), in men with recurrent disease and (“LIGHTHOUSE,” NCT04186819), in men with newly diagnosed prostate cancer. Currently, rhPSMA compounds have not received regulatory approval.
For more information: www.blueearthdiagnostics.com


 February 03, 2026
February 03, 2026 









