A new study published in JCO Clinical Cancer Informatics tackles a critical challenge in cancer diagnostics: ensuring that artificial intelligence (AI) performs equitably across racially diverse patient populations. This research marks a milestone in prostate cancer care, presenting the first large-scale comparative analysis of a digital pathology AI prognostic model in both African American and non–African American patients. The findings offer key insights into the performance of AI in addressing bias in oncologic outcomes.
Prostate cancer is the second-leading cause of cancer death in American men.1 Black men face a disproportionately high burden of prostate cancer —nearly twice the incidence and more than double the mortality rate compared to white men.2 Yet, they remain underrepresented in clinical trials and often receive less aggressive treatment.3 The disparity has long raised concerns about representation in clinical research and whether AI algorithms might inadvertently reflect systemic biases residing in historical data.
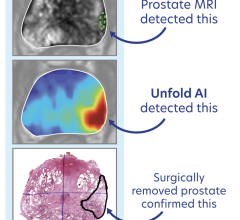
A study led by Mack Roach III, M.D., Professor of Radiation Oncology and Urology at the University of California San Francisco (UCSF), based on pooled data from randomized clinicals evaluated the performance of a multimodal artificial intelligence (MMAI) model developed by Artera to close gaps in care and validate that AI can be applied ethically and equitably.
Artera, a precision medicine company, engineered MMAI to provide prognostic and predictive outcomes in men with localized prostate cancer. Specifically, the ArteraAI Prostate Test uses a proprietary algorithm to digitize and analyze tissue from a patient’s biopsy alongside clinical data from the NCI-sponsored tissue bank housed at UCSF on behalf of the NRG. These AI biomarker results — validated by numerous phase three randomized trials with racially diverse clinical datasets — inform oncologists whether a patient is likely to benefit from a particular therapy.

The study analyzed 5,708 prostate cancer patients from five phase three clinical trials — including 948 African American men — a considerable sample size in this context. The analysis focused on Artera’s prognostic MMAI model to predict both distant metastasis (DM) and prostate cancer-specific mortality in patients (PCSM).
Both endpoints demonstrated strong, nearly identical predictive accuracy across racial groups, with no evidence of algorithmic bias, substantiating MMAI’s clinical utility across racial subgroups and its potential to support equitable, personalized treatment.
Success does bring skepticism, and as AI becomes increasingly integrated into clinical care, concerns will likely arise regarding bias, equity, and data privacy. To address concerns, the research team partnered with global organizations to ensure that its model utilizes diverse datasets for training and validation, thereby advancing more equitable and representative tools for prostate cancer care.
Moreover, unlike population-level registries (such as SEER), this research used data from randomized, controlled trials, accounting for the consistency and quality of care, treatment, and follow-up.
“The technology has been validated through rigorous prostate cancer clinical trials and is now FDA-compliant. With the results, MMAI represents the tip of the iceberg in precision oncology,” said Dr. Roach. “It moves us beyond diagnosis — enabling more personalized treatment by identifying which patients are most likely to benefit from aggressive therapies.”
Other benefits of MMAI include avoiding overtreatment — such as unnecessary hormone therapy — while ensuring that high-risk patients receive timely care. The technology can also detect patterns that human pathologists might not see, elevating the quality of care across all demographics.
“We’re thrilled to have this important validation study published as we continue our ongoing validation efforts across additional racial subgroups,” said Timothy Showalter, Chief Medical Officer at Artera. “As the leader in leveraging AI for more personalized care, this work is crucial to ensure our tools are broadly representative.”
Artera’s test is currently available through its CLIA-certified and CAP-accredited lab in Jacksonville, Florida. As more institutions integrate AI into their diagnostic workflows, this study could serve as a touchstone, showing that equity and innovation need not be mutually exclusive.
“It’s important to conduct studies to ensure new clinical decision support tools work well across a diverse patient population,” said Dr. Roach, “This study goes beyond claims and offers evidence that we can develop and validate AI tools that perform responsibly and with no bias. Our results also set the stage for addressing disparities with AI across a broader range of conditions.”
For more information, please visit https://artera.ai/
- https://www.cancer.org/cancer/types/prostate-cancer.html
- https://zerocancer.org/black-men#statistics
- https://www.prostatehealthed.org/phen_Detail.php?News=2022


 December 04, 2025
December 04, 2025