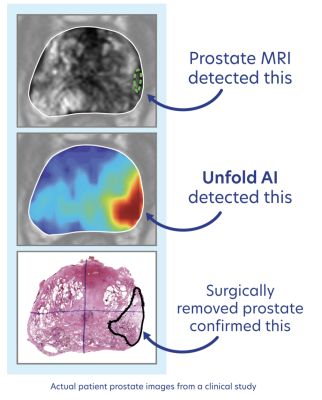
June 17, 2024 — Avenda Health, an AI healthcare company creating the future of personalized prostate cancer care, announces the findings of its new study published in The Journal of Urology, demonstrating the ability of artificial intelligence (AI) to accurately identify cancer in oncology imaging and diagnostics. Titled, “Artificial Intelligence Improves the Ability of Physicians to Identify Prostate Cancer Extent,” the study found AI-assisted cancer identification with Unfold AI, Avenda Health’s AI-powered cancer mapping technology, improved urologists and radiologists abilities in identifying cancer extent by 45x.
In this groundbreaking study, researchers compared the performance of physicians when using AI compared to when not using AI to create cancer margins that encompass all clinically significant prostate cancer while minimizing non-cancerous tissue within the margin. The multi-reader, multi-case study involved seven urologists and three radiologists from five different institutions and spanning decades of experience, assessing 50 different prostate cancer cases each.
The readers initially defined cancer margins manually, and after a period of at least four weeks, used AI software to define cancer margins. The study found AI-assisted cancer margins significantly outperformed cognitively-defined and hemi-gland margins, with a balanced accuracy of 84.7 percent compared to 67.2 percent and 75.9 percent, respectively.
Importantly, AI-assisted margins reduced the underestimation of cancer extent, with a negative margin rate of 72.8 percent compared to 1.6 percent for cognitively-defined margins. The results show Unfold AI can enhance accuracy in defining prostate cancer margins, leading to improved patient outcomes by informing better treatment strategies.
Authored by Sakina Mohammed Mota, PhD and Alan Priester, PhD, and co-authored by, Joshua Shubert, Jeremy Bong, James Sayre, PhD, Brittany Berry-Pusey, PhD, Wayne G Brisbane, MD, and Shyam Natarajan, PhD, this study demonstrates an AI-focused approach has the potential to elevate the efficacy of interventions, such as focal therapy, offering a significant advancement in prostate cancer management.
“This study is important because it shows the ability of AI to not only replicate expert physicians, but to go beyond human ability,” said Dr. Wayne Brisbane. “By increasing the accuracy of cancer identification in the prostate, more precise and effective treatment methods can be prescribed for patients.”
Unfold AI was just included in the American Medical Association’s (AMA) 2024 Current Procedural Terminology (CPT) code set. As a Category III code, Unfold AI will now be billable by insurance, improving access to this personalized prostate cancer care.
“It’s empowering for physicians to see this kind of innovation being validated through studies and recognized by the AMA,” said Avenda Health CEO Dr. Shyam Natarajan. “Avenda Health is at the forefront of using AI to vastly improve tools at physicians' disposal to more effectively treat patients.”
For more information: avendahealth.com


 March 06, 2026
March 06, 2026 









