Image courtesy of Avenda Health
March 21, 2024 — Avenda Health, an AI healthcare company creating the future of personalized prostate cancer care, announced its FDA cleared prostate cancer management platform, Unfold AI, is being used for the first time in a commercial setting with patients at a renowned U.S. research hospital. Several physicians at the hospital, including Wayne Brisbane, MD, are using the technology.
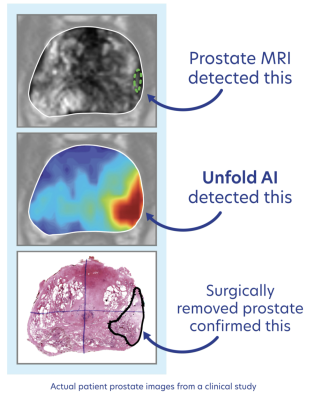
Avenda Health's Unfold AI platform is being used to support physicians and patients with treatment selection, planning, guidance, and follow-up. It combines patient-specific data from prostate imaging, biopsies, and pathology into deep-learning algorithms to create a unique and tailored cancer estimation map. Its 3D, AI-generated map visualizes the location of the cancer for physicians to use in treatment decision making and interventional planning.
"This is a major step forward for the prostate cancer community and we are beyond thrilled to see a decade of hard work and research pay off," said Dr. Leonard Marks, co-founder and Chief Medical Officer of Avenda Health. "Our mission at Avenda Health is to create a better quality of life for prostate cancer patients and to give urologists a clearer view of the cancer so we can better treat our patients. Unfold AI will improve clinical care and decision making."
Validated in multiple clinical studies, urologists have improved their sensitivity of identifying the extent of the tumor to over 98% with Unfold AI. In clinical trials, guidance from the platform caused physicians to change their treatment recommendations in 28% of the cases, many times towards a more localized treatment. Based on the results provided by Unfold AI, multiple treatment options can be chosen including active surveillance, whole gland treatments, like radical prostatectomy and radiation therapy, or a soft tissue laser ablation using a device from Avenda Health — FocalPoint.
"With a wide breadth of ablative options available, Unfold AI represents the first technology to improve tumor localization and patient selection," said Wayne Brisbane, M.D., assistant professor of urology. "I am hopeful that Unfold AI will be to intraprostatic staging what prostate-specific membrane antigen PET-CT has been for extraprostatic staging. Additionally, there are potential applications for surgery, radiation therapy, and patient decision-making."
In December of 2022, Unfold AI received 510(k) clearance by the US FDA.
For more information: avendahealth.com.


 February 05, 2026
February 05, 2026 









