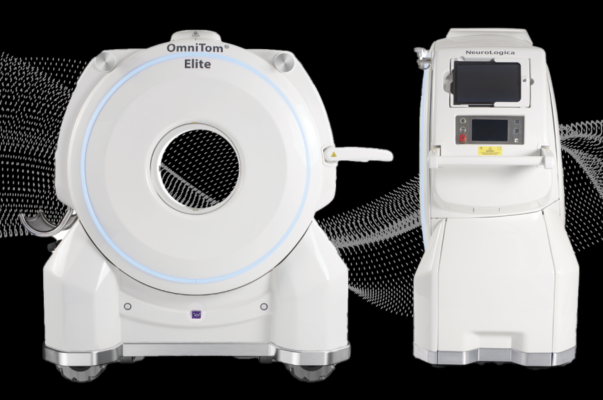
July 15, 2024 — NeuroLogica Corp, a subsidiary of Samsung Electronics Co. Ltd., announced its latest configuration of the mobile computed tomography (CT) OmniTom Elite with Photon Counting Detector (PCD) technology has received U.S. Food and Drug Administration (FDA) 510(k) clearance. This advancement builds upon the existing PCD capabilities with groundbreaking enhancements, further solidifying OmniTom Elite's position at the forefront of mobile CT imaging technology.
The newly cleared features optimized for PCD include several significant innovations:
- Ultra-High-Resolution Mode: This development promises unprecedented clarity and detail in CT imaging. The addition of an ultra-high-resolution mode now offers an impressive 0.141 mm resolution.
- Advanced Image Processing Algorithm: The OmniTom Elite now supports post-reconstruction features such as Virtual Non-Contrast (VNC) and bone removal, enhancing diagnostic capabilities and image quality.
- Expanded Scanning Scope: The PCD scanning capabilities now cover a full 30cm field of view, ensuring comprehensive imaging potential for a variety of clinical applications.
- Helical Scanning Capability: This new feature allows for continuous spiral scanning, providing a faster and more efficient imaging process.
- Enhanced Operating Environment: The operating environment ambient temperature has been increased, broadening the range of conditions in which the device can be used.
These enhancements mark a significant step forward from the initial PCD configuration, which was already a pioneering achievement in mobile CT technology. The original OmniTom Elite with PCD was the first FDA 510(k) cleared, single-source photon counting computed tomography scanner with a single detector, capable of generating spectral CT images at multiple energy levels. This enhancement marks the third 510(k) clearance for OmniTom Elite PCD, speaking to NeuroLogica’s commitment to continuous innovation.
"Samsung and NeuroLogica's technological innovation has resulted in the FDA clearance of OmniTom Elite PCD," said Kyu-Tae Yoo, Head of Samsung Electronics' Medical Device Business and Samsung Medison CEO. "We will continue to develop leading technologies to improve medical staff's convenience and diagnostic accuracy.
Renaud Maloberti, Vice President & Head of mCT Business, NeuroLogica, commented, "Our commitment to innovation drives us to continually improve our products. With these new features, the OmniTom Elite PCD not only offers exquisite image quality and diagnostic capabilities but also ensures a more versatile and efficient imaging process. This is a significant advancement for point-of-care imaging, continuing our commitment to enable clinicians to make a more confident diagnosis due to the improved clinical information."
The University of Dundee and Massachusetts General Hospital have partnered with NeuroLogica to collaborate on research aimed at pushing the boundaries of medical imaging in healthcare using OmniTom Elite PCD.
Existing OmniTom Elite customers can "UpTrade" their scanners to the OmniTom Elite PCD, granting them access to the latest advancements in photon counting technology.
For more information: www.samsung.com


 March 06, 2026
March 06, 2026 









