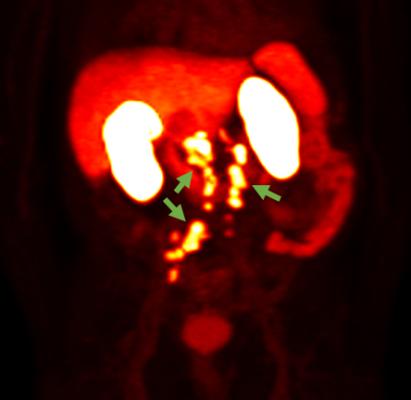
May 16, 2022 — Blue Earth Diagnostics, a Bracco company and recognized leader in the development and commercialization of innovative PET radiopharmaceuticals, announced results of additional endpoints from its Phase 3 SPOTLIGHT trial of 18F-rhPSMA-7.3 in recurrent prostate cancer. Results reporting the impact of 18F-rhPSMA-7.3 PET on upstaging patients were reported in a Late-breaking Abstract oral presentation at the 2022 AUA Annual Meeting (AUA2022). 18F-rhPSMA-7.3 is an investigational Prostate-Specific Membrane Antigen-targeted radiohybrid (rh) PET imaging agent.
“The ability to determine the extent and location of recurrent prostate cancer to inform appropriate clinical management is key for physicians and their patients, as up to 40% of patients who undergo radical prostatectomy, and up to 50% of patients who undergo radiation therapy will develop local or distant recurrences within 10 years,” said Mark T. Fleming, MD, Virginia Oncology Associates, US Oncology Research, Norfolk, Va., on behalf of the SPOTLIGHT Study Group. “Conventional imaging techniques have many limitations in prostate cancer identification and localization, and greater imaging accuracy is needed throughout the care continuum to optimize therapeutic decision-making. These findings from the SPOTLIGHT study showed that 45 - 47% (113 - 117/250) of patients identified as negative on conventional baseline had at least one True Positive (confirmed by Standard of Truth) after lesion identified by 18F-rhPSMA-7.3 PET. This frequently resulted in post-scan upstaging, particularly among patients with intact prostates. Actionable information such as this may help to define sites of disease recurrence and inform salvage therapy decisions.”
“These results from the Phase 3 SPOTLIGHT trial are part of a New Drug Application with the U.S. Food and Drug Administration (FDA) for 18F-rhPSMA-7.3 PET imaging, and we are pleased that they are being presented to the clinical community at the prestigious AUA2022 conference,” said David E. Gauden, D.Phil., Chief Executive Officer of Blue Earth Diagnostics. “In line with our mission to help patients with cancer, we continue to develop our uniquely comprehensive prostate cancer portfolio, which includes 18F-fluciclovine and investigational rhPSMA compounds for potential use in diagnostic PET imaging and targeted radiopharmaceutical therapy. 18F-rhPSMA-7.3 represents a new class of high affinity PSMA-targeted PET radiopharmaceuticals. Early studies of 18F-rhPSMA-7.3 demonstrated high binding affinity for PSMA, together with biodistribution data suggesting the potential for low bladder activity.”
The SPOTLIGHT trial (NCT04186845) is a Phase 3, multi-center, single-arm imaging study conducted in the United States and Europe to evaluate the safety and diagnostic performance of 18F-rhPSMA-7.3 PET imaging in men with suspected prostate cancer recurrence based on elevated PSA following prior therapy. Key results for 18F-rhPSMA-7.3 PET were previously presented at ASCO GU in February 2022.1
The findings presented at AUA2022 included Correct Detection Rate (CDR) assessment (the percentage of all patients scanned with at least one true positive PET finding as compared to the Standard of Truth of histopathology or confirmatory conventional imaging), and its impact on patient upstaging. They were based on individual read results from three blinded, independent PET readers. In total, the Efficacy Analysis Population (EAP) of 366 men had a composite Standard of Truth. Among EAP patients, 68% (250/366) had negative baseline conventional imaging. Among the 250 patients with negative baseline conventional imaging, 18F-rhPSMA-7.3 showed a CDR of 45─47% (113 - 117/250) across the three readers.
Among patients who had undergone prostatectomy, 3.5-8.0% (7-16/201) of 18F-rhPSMA-7.3 positive scans showed lesions in the prostate bed region, with 18-21% (36-43/201) in pelvic lymph nodes and 21-26% (43-52/201) in other sites that led to upstaging. Among patients who had received radiotherapy, these values were 39-41% (18-19/46), 6.5% (3/46) and 20-30% (9-14/46), respectively. Very few patients had an alternative primary therapy and no definitive conclusions could be drawn for them.
The Late-breaking Abstract was discussed in an oral Plenary Session presentation at AUA2022 on May 13, 2022, “Impact of 18F-rhPSMA-7.3 PET on upstaging of patients with prostate cancer recurrence: results from the prospective, Phase 3, multicenter SPOTLIGHT study,” by Mark T. Fleming, MD, Virginia Oncology Associates, U.S. Oncology Research, Norfolk, Va., on behalf of the SPOTLIGHT Study Group. The Journal of Urology abstract is available here.
Related content:
Blue Earth Diagnostics Announces Key Results from Phase 3 SPOTLIGHT Study of 18F-rhPSMA-7.3


 February 26, 2026
February 26, 2026 









