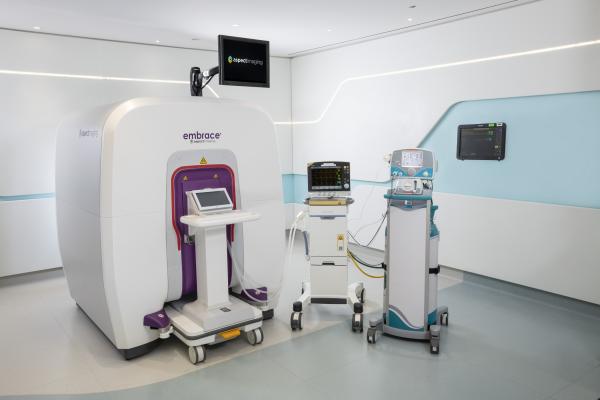
December 1, 2020 — Based on its recent analysis of the global neonatal intensive care unit magnetic resonance imaging (ICU MRI) market, Frost & Sullivan recognized Aspect Imaging Ltd. with the 2020 Global Technology Innovation Leadership Award. Aspect Imaging's Embrace Neonatal MRI System [Embrace] is the only FDA-cleared and CE-marked disruptive MRI system designed specifically to perform MRI scans on critically ill babies in the NICU, eliminating the stress, infection risks and challenges of transporting fragile patients to the Radiology department.
"Recognizing the challenges involved in transporting neonates from a NICU to a radiology unit or department, Aspect Imaging developed a first-of-its-kind MRI system that can be used safely on a premature or ill newborn," said Ashish Kaul, Senior Research Analyst. "Embrace can deliver high-resolution 2D and 3D images, greatly enhancing diagnosis and patient care."
Embrace is a fully enclosed system utilizing Aspect Imaging’s proprietary magnet technology that does not require any cooling and consumes less power than traditional MRI systems. The solution can be used for babies weighing between 1 and 4.5 kilograms, with a maximum head circumference of 38 centimeters. Embrace allows users to set a controlled environment temperature of up to 36.5 degrees Celsius within the Embrace patient bed, critical for premature patients. The low sound profile of the system, combined with the controlled temperature environment and the lack of transport requirement, help ensure little-to-no body movement during the scanning procedure, reducing the need for sedation. This revolutionary system is fully self-shielded eliminating the need for a special safety zone or RF-shielded room, enabling installation inside the NICU.
Embrace reduces parents’ emotional anxiety by allowing them to be present in the room and view their baby on a full-color video display during the scanning procedure. In addition, Embrace’s operating and maintenance costs are significantly lower than the cost of conventional superconductor MRI systems. The system has received FDA clearance and the CE mark and is currently used for clinical and research purposes in hospitals in the US and around the world. By being placed inside the NICU, the Embrace allows the medical and nursing team to obtain neuro-images when they need them without leaving the specialty care environment, assuring users of quality care for babies before, during, and after the procedure in the NICU.
"Aspect Imaging supports neonatologists and neuroradiologists in making accurate diagnostic decisions by delivering clinical information to them easily via connected picture archiving and communication system (PACS) servers, without the disruption of transporting babies out of the NICU," noted Kaul. "The company aims to disrupt and redefine the entire neonatal medical imaging space and has shown strong growth potential across geographic regions."
Each year, Frost & Sullivan presents this award to the company that has demonstrated uniqueness in developing and leveraging new technologies that deliver significant customer value.
Frost & Sullivan Best Practices Awards recognize companies in a variety of regional and global markets for demonstrating outstanding achievement and superior performance in areas such as leadership, technological innovation, customer service, and strategic product development. Industry analysts compare market participants and measure performance through in-depth interviews, analyses, and extensive secondary research to identify best practices in the industry.
For more information: www.aspectimaging.com


 February 16, 2026
February 16, 2026 









