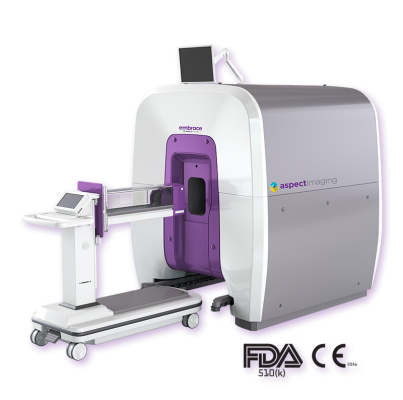
November 25, 2019 — Embrace Neonatal MRI is a U.S. Food and Drug Administration (FDA) cleared and CE marked compact magnetic resononance imaging (MRI) system ergonomically designed to fit inside the neonatal intensive care unit (NICU). This point-of-care approach keeps critically ill infants safe in a temperature-controlled environment, eliminating the risk of transporting delicate newborns to an external MRI system. The customized patient bed and RF head coil accommodates intubated babies with routing mechanisms to allow safe tube and line management. To reduce noise exposure, the Embrace system is significantly quieter than conventional MRI and does not require a special safety zone or special RF-shielded room. Integrating the Embrace System in the NICU helps reduce procedure workflow and mitigates schedule disruption.
For more information: www.embracemri.com


 February 13, 2026
February 13, 2026 









