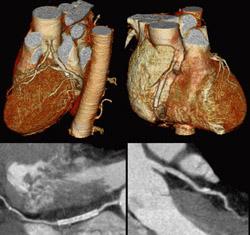
August 18, 2010 - Cardiac computed tomography (CT) was found to be safe, accurate, and cost-effective in low risk patients in a recent scientific statement published by the American Heart Association (AHA), titled “Testing of Low-Risk Patients Presenting to the Emergency Department With Chest Pain.” The paper summarizes the use of cardiac CT angiography as “Computed tomography coronary angiography has also shown promise in this setting. A negative accelerated diagnostic protocol evaluation allows discharge, whereas patients with positive findings are admitted. This approach has been found to be safe, accurate, and cost-effective in low-risk patients presenting with chest pain.” The use of CT angiography is rapidly expanding, primarily due to well-published studies demonstrating cost effectiveness and safety in this population. Hospitals and cardiologists are increasingly turning to this test to determine who of those presenting to the emergency room can be safely discharged from the hospital in a timely fashion. New, large randomized studies that were already presented, but not yet published, further reinforce the utility of this modality and the safety, efficacy and cost savings compared to other imaging tests. These guidelines, in association with NICE guidelines recently published on the same topic, reiterate the important and growing role of cardiac CT angiography and calcium scoring as rapid methods to triage patients in the emergency department safely and most cost-effectively than other imaging modalities," said Matthew J. Budoff, M.D., FSCCT, professor of medicine at the David Geffen School of Medicine at UCLA, director of cardiac CT at Los Angeles Biomedical Research Center at Harbor UCLA Medical Center in Torrance, CA and president of the Society of Cardiovascular Computed Tomography (SCCT). For more information: www.SCCT.org


 February 13, 2026
February 13, 2026 









