August 14, 2014 — Toshiba Medical Systems Europe will introduce the SURE Subtraction Coronary software at the 2014 European Society of Cardiology (ESC) Congress in Barcelona, to be held Aug. 30 to Sept. 3. The software was developed in close cooperation with the Iwate Medical University in Japan, leading hospitals in the United States and Europe, and the European-based research center Toshiba Medical Visualization Systems.
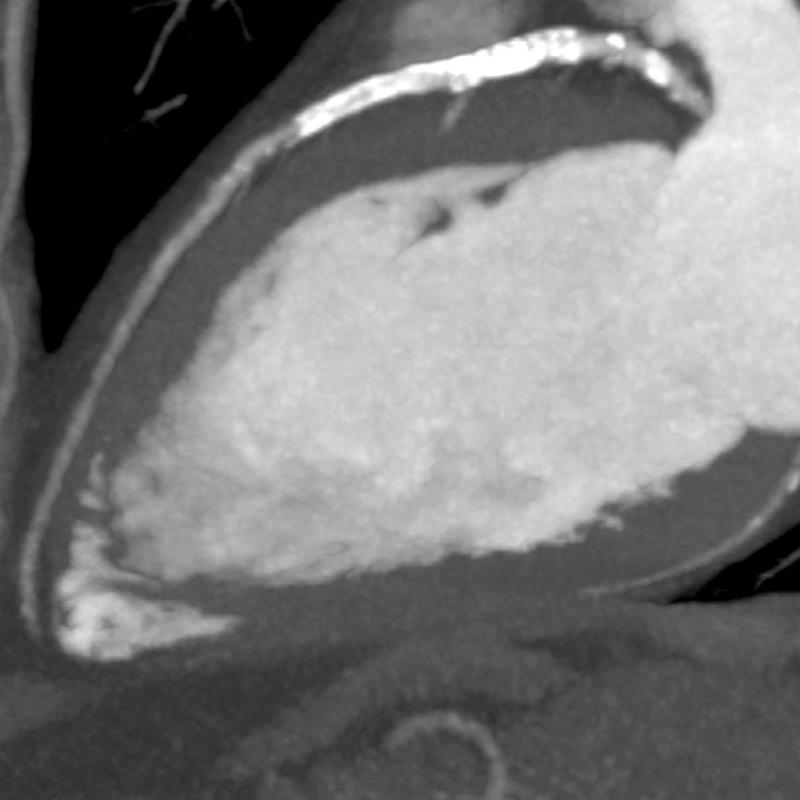
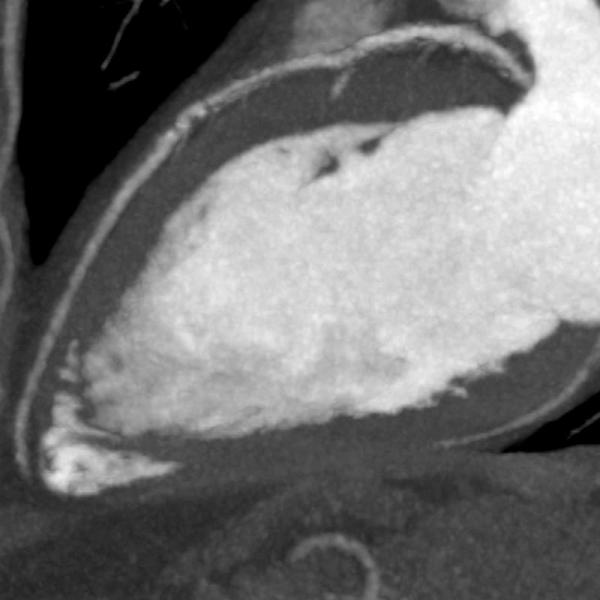
Visualization of the coronary lumen can be improved by advanced subtraction software. The release represents a major step forward in diagnosing coronary artery disease (CAD) in patients with severe calcium or stents for whom cardiac CT (computed tomography) angiography (CTA) was not recommended.
Severe coronary calcification influences the effectiveness of coronary CTA in ruling out CAD. Frequently these patients are referred for invasive angiography because of clinical suspicion of significant CAD. Stents placed in the coronary arteries can make visualization of the lumen within the stent difficult, hindering the ability to diagnose in-stent restenosis. SURE Subtraction Coronary removes calcification and stents from the coronary arteries, therefore improving visualization of the coronary lumen. Blooming effects caused by calcification are dramatically reduced.
An added benefit of SURE Subtraction Coronary is that it can be obtained with a near-dose-neutral scanning protocol. Coronary Subtraction is performed by subtracting a routine calcium score dataset from a coronary CTA dataset, the calcium score scan being used as the non-contrast mask for subtraction. Atlas-based cardiac segmentation and sophisticated rigid and deformable registration algorithms enable accurate subtraction of the coronary arteries to become a reality, leading to improved visualization of the coronary lumen.
Participants at ESC are invited to join a satellite lunch symposium Sunday, Aug. 31, to learn more about he new technology. Details can be found at www.toshiba-medical.eu/eu/events/esc-2014-workshop-registration.
For more information: www.toshiba-medical.eu


 February 09, 2026
February 09, 2026 









